Session Information
Date: Tuesday, November 12, 2019
Title: Miscellanous Rheumatic & Inflammatory Disease Poster III: Autoimmune Conditions and Therapies
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Immune checkpoint inhibitors (ICI) have revolutionized cancer therapy by harnessing the immune system to fight cancer. They are associated with the development of autoimmune toxicities, referred to as immune-related adverse events (irAE). Rheumatic irAE (Rh-irAE), particularly arthralgias and arthritis are common and optimal management remains unknown. The Canadian Research Group of Rheumatology in Immuno-Oncology (CanRIO) is an emerging network of Canadian rheumatologists with an interest in Rh-irAE secondary to ICI.
Methods: Patients presenting with rheumatic symptoms associated with ICI therapy between 2013 and January 2019 at participating CanRIO sites were identified. Cases were stratified based on the presence or absence of pre-existing autoimmune disease (PAD). Standardized data were extracted by chart review. The data were pooled and analyzed descriptively.
Results: A total of 118 patients without PAD who developed 140 Rh-irAE were identified, 59% were male with a mean age of 62 years. The most common indications for ICI were melanoma (n=57, 48.3%), lung (n=30, 25.4%), and genitourinary (n=19, 16.1%) with stage 4 disease seen in 79%. ICI included nivolumab (n=30, 25.6%), pembrolizumab(n=38, 32.5%), ipilimumab (n=1, 0.9%), durvalumab (n=3, 2.6%), atezolizumab (n=4, 3.4%) or combination therapy (n=29, 24.8%). Common Rh-irAE included symmetric polyarthritis (n=45, 32%), arthralgias/myalgias or acute flare of osteoarthritis (n=20, 16.9%), polymyalgia rheumatica-like presentation (n=17, 12%), tenosynovitis/enthesitis (n=17, 12%), sicca (n=11, 9.3%) and myositis (n=9, 7.6%).
The majority of cases experienced at least one other non-rheumatic irAE (O-irAE) (n=63, 53%). Mean time from first ICI exposure to onset of Rh-irAE was 6.8 months. ICI was either held or discontinued in 65% (n=77). Despite this, (n=81, 68.6%) had favorable tumor response, while (n=14, 12%) had tumor progression. There were no deaths related to Rh-irAE.
The majority of patients without PAD had either a partial or complete response to either mono- or combination therapy with oral prednisone (n=77; maximum dose 60 mg/d), non-steroidal anti-inflammatories (NSAID; n=52), intra-articular corticosteroids (n=29), hydroxychloroquine (n=26), methotrexate (n=17), or tumor necrosis factor alpha inhibitors (n=9).
Twenty patients with pre-existing autoimmune diseases were identified 70% of whom were in remission prior to starting ICI. 50% (n=10) had flares, 15% (n=3) developed a new Rh-IrAE and 20% (n=4) developed O-irAE.
Conclusion: This is the largest multi-centered cohort of Rh-irAE described to date. Seronegative inflammatory polyarthritis was the most common Rh-IrAE although a broad range of conditions were identified. The most common first-line treatment was systemic corticosteroids followed by NSAID and intra-articular steroid injections. Prednisone was effective, however high doses were required. Disease-modifying antirheumatic drugs (DMARD) and biologic therapy were well tolerated and effective, with hydroxychloroquine being the most commonly used DMARD. Despite moderate to high doses of immunosuppression, the majority of patients had favorable tumor responses.
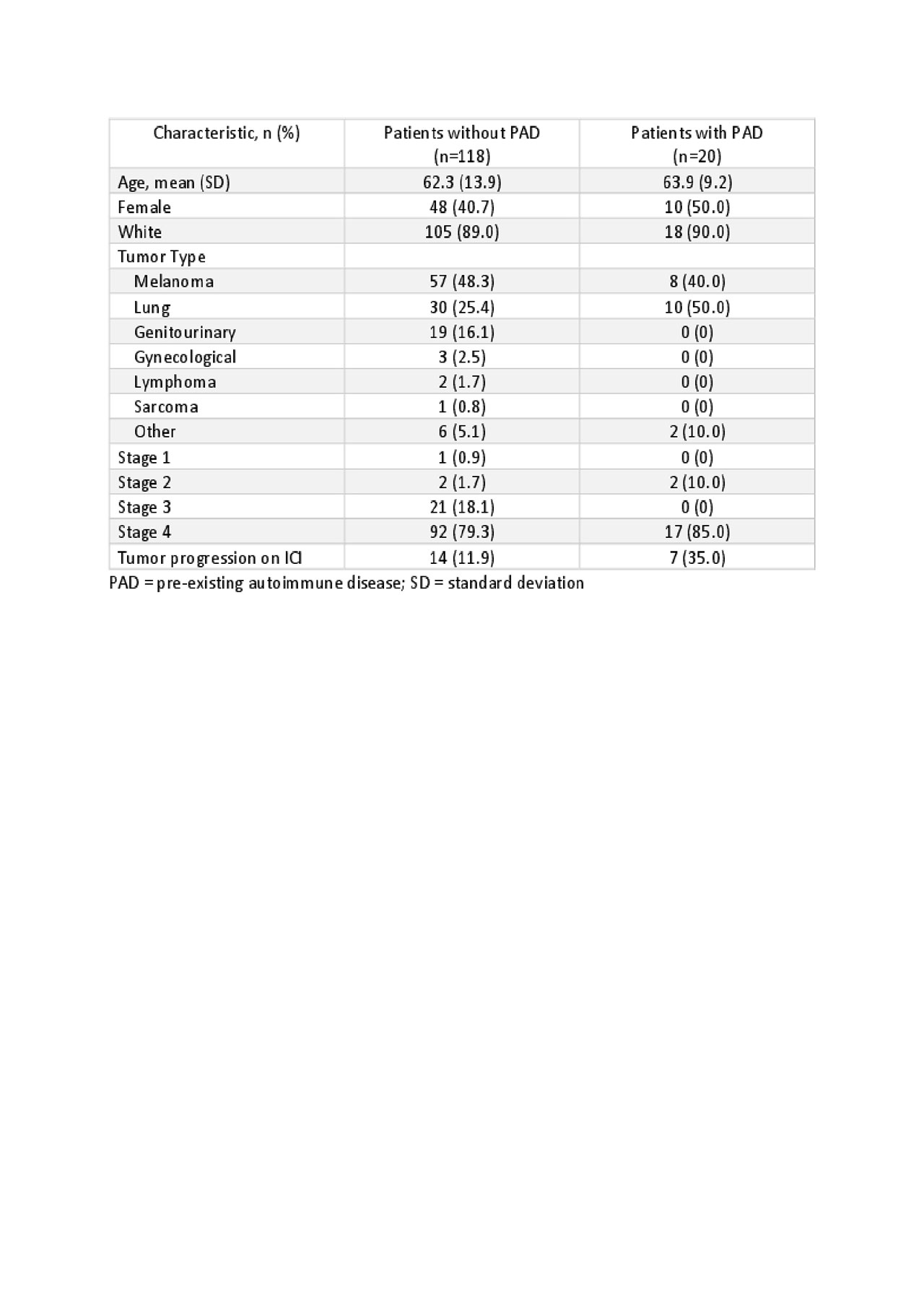
Table 1- Demographics and tumor type

Table 2 Rh-irAE and selected investigations

Table 3 Effects of ICI on pre-existing autoimmune disease
To cite this abstract in AMA style:
Roberts J, Ennis D, Hudson M, Ye C, Saltman A, Hoa S, Pope J, Himmel M, Maltez N, Fifi-Mah A, Tisseverasinghe A, Rottapel R, Al Jumaily K, Ly C, Cartagena L, Jamal S. Rheumatic Immune Related Adverse Events Associated with Cancer Immunotherapy: A Nationwide Multi-centre Canadian Cohort from the Canadian Research Group of Rheumatology in Immuno-oncology (CanRIO) [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/rheumatic-immune-related-adverse-events-associated-with-cancer-immunotherapy-a-nationwide-multi-centre-canadian-cohort-from-the-canadian-research-group-of-rheumatology-in-immuno-oncology-canrio/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rheumatic-immune-related-adverse-events-associated-with-cancer-immunotherapy-a-nationwide-multi-centre-canadian-cohort-from-the-canadian-research-group-of-rheumatology-in-immuno-oncology-canrio/
