Session Information
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: To evaluate ultrasound (US) as a diagnostic tool for gout with positive urate crystal microscopy as the gold standard, using the OMERACT US working group definitions for crystal deposits.
Methods: US examinations (28 joints, 26 tendons) were performed in patients with clinically suspected gout. Joints (metacarpophalangeal, wrist, elbow, metatarsophalangeal, tibiotalar and knee joints) and tendons (extensor tendons of the wrist (scored as compartments (1-6)), triceps, quadriceps, patella, peroneus (longus and brevis scored as one), tibialis posterior and Achilles) were evaluated for the OMERACT gout structural lesions (double contour (DC), tophus, aggregates and erosions). Each of the structural lesions were registered as present/absent for each patient. The US assessment was compared to the gold standard reference for gout: presence/absence of monosodium-urate (MSU) crystals at joint fluid microscopy. The microscopies were performed by a rheumatologist blinded to US findings.
The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of each US lesion, with microscopy as gold standard, were evaluated.
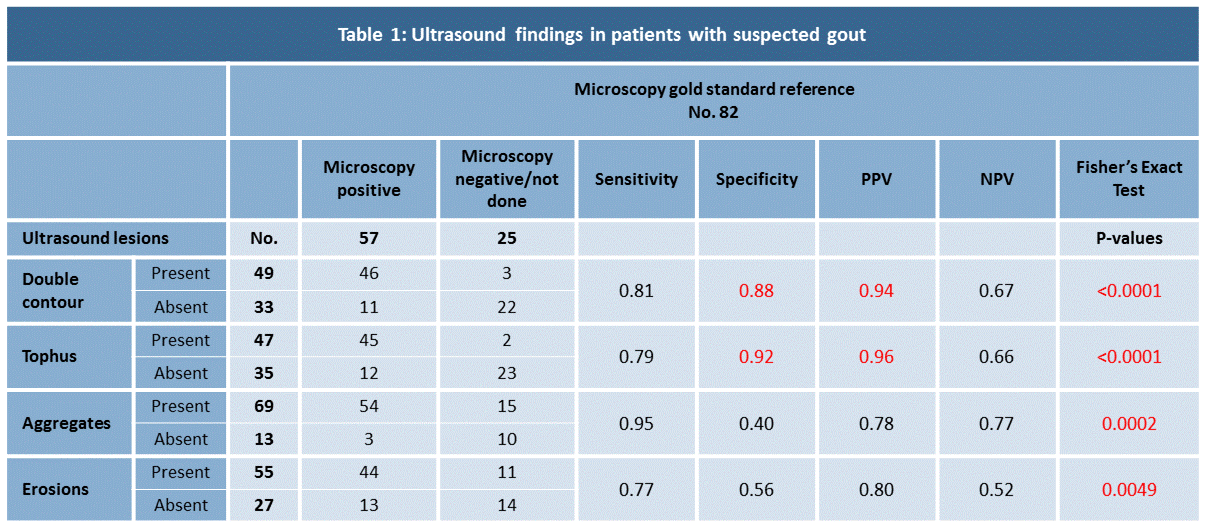
Results: 82 patients (70 males, 12 females), mean age of 61 years (19-88) were included. 57 had a positive microscopy for MSU crystals, 23 patients had a negative microscopy and in 2 patients joint aspiration was not possible (table 1).
All four structural lesions were statistically significant more frequent in patients with positive MSU microscopy compared to patients with negative microscopy (Fisher’s exact test with p-values from < 0.0001 to 0.0049), and all lesions were found to have high sensitivities for gout (ranges from 0.77-0.95). DC and tophus showed high specificities for patients with microscopically verified gout (0.88 and 0.92, respectively) and were also found to have high PPV (0.94 and 0.96, respectively). Low specificities were found for both aggregates and erosions (0.40 and 0.56). Negative predictive values were low to moderate for all lesions (ranges from 0.52-0.77).
Conclusion: The OMERACT set of US definitions of structural gout lesions seems to be a valid tool for diagnosis of gout in clinical practice. Particularly, DC and tophi seem to have a high specificity and high PPV for the disease, when joint fluid microscopy is considered as gold standard.
To cite this abstract in AMA style:
Christiansen S, Østergaard M, Slot O, Terslev L. Evaluating the OMERACT Definitions of Ultrasound Gout Structural Lesions in the Diagnosis of Gout [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/evaluating-the-omeract-definitions-of-ultrasound-gout-structural-lesions-in-the-diagnosis-of-gout/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluating-the-omeract-definitions-of-ultrasound-gout-structural-lesions-in-the-diagnosis-of-gout/