Session Information
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Disease flare despite high cumulative glucocorticoid exposure is one of the hallmarks of giant cell arteritis (GCA). Tocilizumab is effective in controlling disease activity in most patients, but up to 35% of them fail this treatment due to inefficacy or side-effects. Thus, additional remission maintenance and glucocorticoid-sparing options are greatly needed in GCA. Interleukins (IL)-12 and 23 are thought to play a pathogenic role in this disease. Therefore, we evaluated the efficacy of the IL-12/23 antagonist ustekinumab (UST) in GCA patients.
Methods: We conducted a prospective, single center, open-label pilot study (ClinicalTrials.gov NCT02955147) to evaluate the efficacy and safety of UST in combination with prednisone for new onset and relapsing GCA patients with active disease. GCA diagnosis required positive temporal artery biopsy or vascular imaging. Active disease for study enrollment was defined as the presence of cranial or PMR signs/symptoms along with elevation of ESR (≥40 mm/hour) or CRP (≥10 mg/L) within 6 weeks of baseline. UST 90 mg was administered subcutaneously at baseline and weeks 4, 12, 20, 28, 36 and 44. All patients received a pre-specified 6-month prednisone taper starting at 60 mg, 40 mg, or 20 mg based on investigators’ clinical judgment. The primary endpoint, prednisone-free remission, was defined as the absence of disease flare from induction of remission up to week 52 and the concurrent normalization of ESR (< 40 mm/hour) and CRP (< 10 mg/L), while adhering to the protocol prednisone taper. Disease flare was defined as the recurrence of signs/symptoms of GCA (e.g., cranial or PMR) that required treatment modification, regardless of ESR and CRP levels. A sensitivity analysis eliminating ESR and CRP from the definition of prednisone-free remission was also completed.
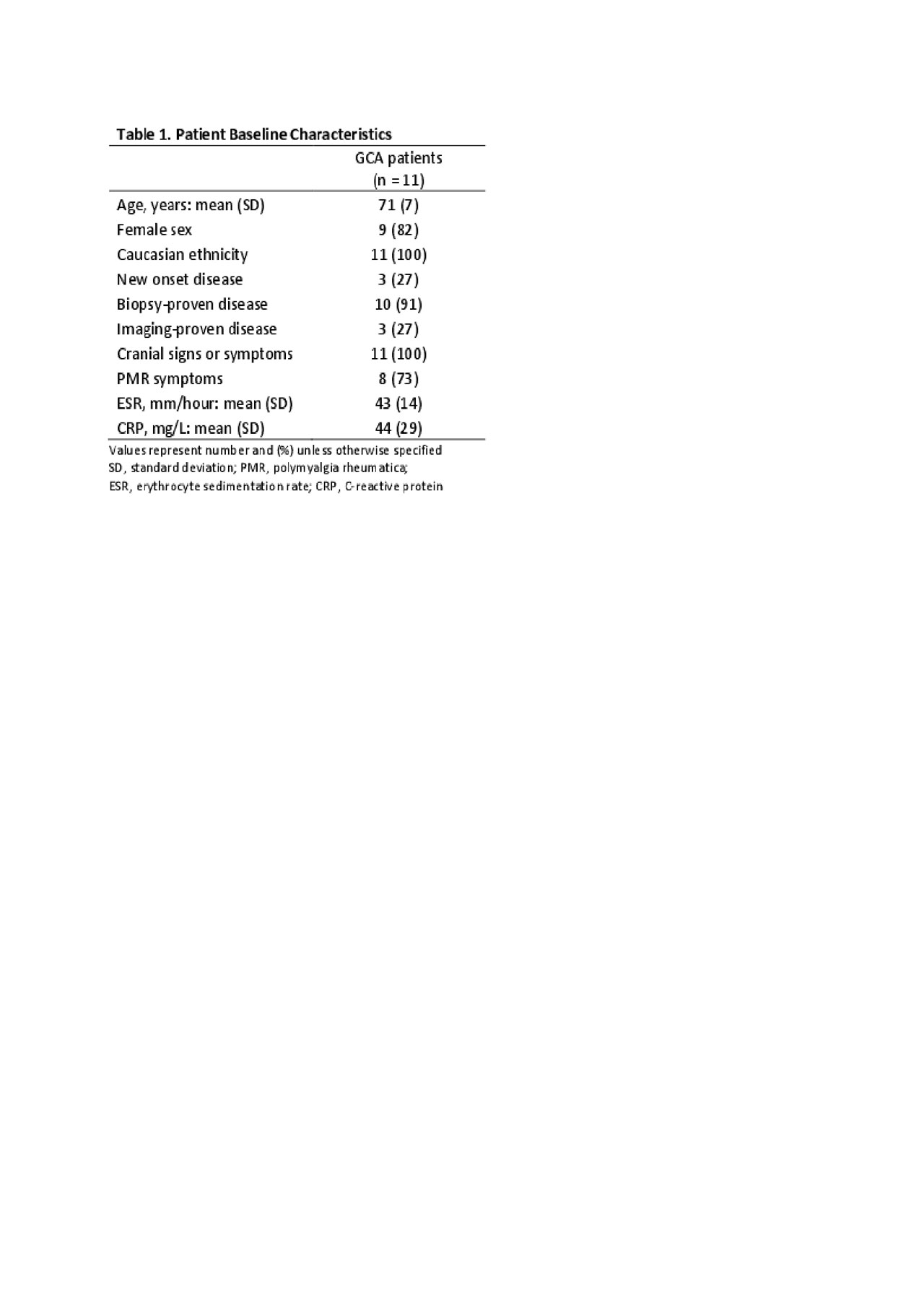
Results: A sample of 20 consecutive patients was planned for the study. However, the study was prematurely terminated due to inefficacy. Here we report the outcomes of the first 11 patients that completed 52 weeks of treatment. Baseline patient characteristics are shown in Table 1. The mean age of the patients was 71 years, 82% were females, 27% had new-onset disease, and 91% had a positive temporal artery biopsy. The initial prednisone dose was 60 mg in 2 patients, 40 mg in 8 patients and 20 mg in 1 patient. Results are shown in Table 2. All patients achieved disease remission within 4 weeks of baseline. Only 2 patients (18%) achieved the primary outcome. Of the 9 patients (82%) who failed to achieve the primary outcome, 7 patients had a flare after a mean period of 23 weeks and 3-6 UST injections. The mean (SD) prednisone dose at the time of flare was 3 (3) mg/day. The other 2 patients who failed to achieve the primary outcome did not have a disease flare, but their inflammatory markers were elevated at week 52. Four patients (36%) met the alternative definition of prednisone-free remission at week 52 (sensitivity analysis). UST was well tolerated. Only 1 patient developed pneumonia, which resolved with oral antibiotics.
Conclusion: UST in combination with 6 months of prednisone was not associated with clinically significant rates of sustained disease remission in this cohort of GCA patients.
To cite this abstract in AMA style:
Matza M, Stone J, Fernandes A, Unizony S. Ustekinumab for the Treatment of Giant Cell Arteritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/ustekinumab-for-the-treatment-of-giant-cell-arteritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ustekinumab-for-the-treatment-of-giant-cell-arteritis/