Session Information
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: The use of immune checkpoint inhibitor (ICI) therapy for malignancy is growing. Various autoimmune and inflammatory syndromes, known as immune-related adverse events (irAEs) have been reported, including inflammatory arthritis (IA). Despite its growing incidence, the clinical features and optimal treatments of IA are not well defined, and long-term outcomes are unknown. This study aims to investigate long-term outcomes of patients (pts) who develop IA due to ICIs and to evaluate those with symptom persistence after ICI cessation, the need for immunosuppressants, and the impact of these medications on underlying malignancy.
Methods: This is a prospective observational study of pts referred to the Johns Hopkins Arthritis Center for IA due to ICIs recruited from 6/2015 to 12/2018 with data available after ICI cessation. ICIs were discontinued for cancer progression, treatment completion, or severe irAEs. Baseline demographics, cancer type, specific ICI treatment, tumor response, personal and family history of autoimmunity, other irAEs, labs, and clinical exam were obtained. Follow up occurred at varying intervals for up to 24 months (mo) from ICI cessation to assess the clinical status of IA and malignancy. Active IA was defined as active joint disease or the need for continued immunosuppression. Kaplan-Meier curves were developed to evaluate pts with persistent IA. A Cox proportional hazards model was used to assess the influence of various factors on IA persistence.
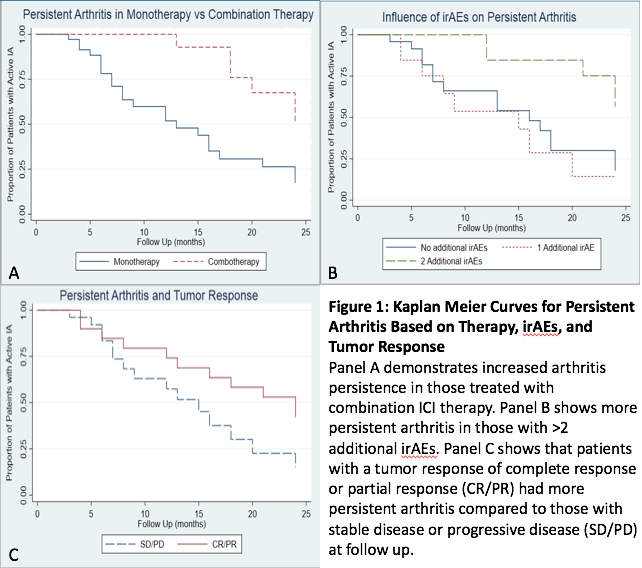
Results: A total of 60pts (53.3% female) were monitored with a median follow up after ICI cessation of 9 mo (1-24 mo). Pts had a wide range of cancers and 50% had other irAEs. Combination ICI therapy was used in 30% of patients. There were low rates of seropositivity (RF 1.8%, CCP 5.5%, ANA 14.3%) and 7pts had a family history of autoimmune disease. Immunosuppressive treatment was required in 75%. Forty-eight pts (80%) were treated with systemic/intraarticular steroids. csDMARDs were used in 19 pts and bDMARDS in 11. A majority (53.1%) had active IA at most recent follow up. Three-month follow up data after ICI cessation was available in 51pts, with 6mo data for 41. At 3mo, 70.6% had active disease; 48.8% had active disease at 6 mo. Among the 20 pts with persistent arthritis at 6 mo, 14 continued to have active disease at last follow up. IA was less likely to improve in those with longer duration of ICI use (hazard ratio 0.93, 95% CI 0.87 to 0.99; p=0.02), in those receiving combination therapy (hazard ratio 0.29, 95% CI 0.12 to 0.72; p=0.008) and in pts with other irAEs (hazard ratio 0.61, 95% CI 0.39 to 0.95, p=0.03). Although not significant, a better tumor response was associated with persistent IA. Tumor response did not appear to be impacted by immunosuppression.
Conclusion: This study demonstrates that a significant number of pts developing ICI-induced IA have persistent arthritis at 3 and 6mo. Specific factors such as longer ICI exposure, combination ICI therapy, and other irAEs increase the risk of persistence. Immunomodulatory treatments were efficacious for symptomatic control while having no apparent effect on tumor response at follow up and persistent arthritis may associate with better tumor response.
To cite this abstract in AMA style:
Braaten T, Brahmer J, Forde P, Le D, Lipson E, Naidoo J, Schollenberger M, Zheng L, Jones M, Shah A, Bingham C, Cappelli L. Immune Checkpoint Inhibitor-Induced Inflammatory Arthritis Persists After Immunotherapy Cessation [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/immune-checkpoint-inhibitor-induced-inflammatory-arthritis-persists-after-immunotherapy-cessation/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immune-checkpoint-inhibitor-induced-inflammatory-arthritis-persists-after-immunotherapy-cessation/