Session Information
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Patients with rheumatoid arthritis (RA) and other systemic diseases (SD) are at an increased risk of developing Herpes Zoster (HZ) due to either the nature of the diseases or the medications used to treat them. Zoster Recombinant Adjuvanted (ZRA) is a novel vaccine that can be used in immunosuppressed populations and was approved by the FDA in 2018. ZRA provides >90% efficacy in non-immunosuppressed patients older than 50. However, concern has been raised that ZRA may trigger disease flares and cause more side effects in immunocompromised patients. We investigated the impact of the new ZRA vaccine in RA and other SD patients, and measured the incidence of flares and side effects.
Methods: We performed a retrospective chart review of patients with RA and SD seen at BWH who received the ZRA vaccine between 2/1/2018 and 2/1/2019. Co-variates of interest were collected. A flare was defined as occurring within 12 weeks of vaccine administration by either: 1) documentation of RA flare in the rheumatologist office notes, telephone encounter or patient portal communication, or 2) new prednisone prescription, or an increase in dose of existing prednisone prescription. Vaccine side effects were defined as muscle soreness or rash at the injection site, redness, mild swelling, fatigue, fevers, myalgias, headaches, nausea, and abdominal pain. All potential flares were independently reviewed, adjudicated, and confirmed by three rheumatologists (EM, MEW, and SD).
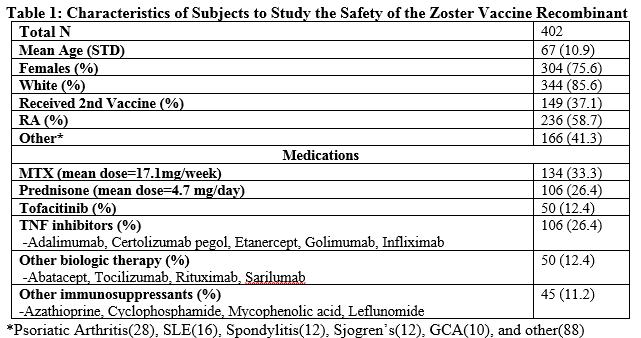
Results: 402 (236 RA and 166 SD) patients who received the new ZRA vaccine between 2/1/2018 and 2/1/2019 were identified. Mean follow up was 13.2 weeks ranging from 1-50 weeks following administration. Patient characteristics are identified in Table 1. We identified 6.7% (n=27) patients who experienced a flare, lower than the observed background six-month flare rate of 30% in RA patients at our center (Bykerk VP et al., 2014). Of these patients 5.7% (n=23) of flares occurred following the first dose and 3.4% (n=5) occurred following the second dose. One patient flared after both doses. All flares were mild, self-limited, responded to treatment with low dose glucocorticoids, and did not warrant a change in immunosuppressive therapy. 13.4% (n=54) patients experienced side effects. Of the patients who experienced side effects, 11.4% (n=46) occurred after the first dose and 8.7% (n=13) occurred following the second dose. Five patients experienced side effects after both doses. All side effects were regarded as mild. No cases of HZ were reported.
Conclusion: In 402 patients who received the new ZRA vaccine, the incidence of disease flares was ≤7% and side effects were 13.4%, which is lower than that observed in the general population. Both flares and side effects were mild, self-limited, and did not require a change in DMARD therapy. No cases of HZ were reported. Formal studies of patients exposed to the vaccine are required to confirm this conclusion. Immunosuppressive therapies may reduce the effectiveness of vaccines. Studies are needed to determine the immune response to the vaccine, and its efficacy in rheumatology patients. We encourage the use of the ZRA vaccine in RA and other SD patients.
To cite this abstract in AMA style:
Stevens E, Weinblatt M, Massarotti E, Griffin F, Emani S, Desai S. Safety of the Zoster Vaccine Recombinant, Adjuvanted in Rheumatoid Arthritis and Other Systemic Rheumatic Disease Patients: A Single Center’s Experience with 400 Patients [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/safety-of-the-zoster-vaccine-recombinant-adjuvanted-in-rheumatoid-arthritis-and-other-systemic-rheumatic-disease-patients-a-single-centers-experience-with-400-patients/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-the-zoster-vaccine-recombinant-adjuvanted-in-rheumatoid-arthritis-and-other-systemic-rheumatic-disease-patients-a-single-centers-experience-with-400-patients/