Session Information
Session Type: Late-Breaking Abstracts
Background/Purpose:
MOR103, a human mAb targeting GM-CSF, is being evaluated for treatment of rheumatoid arthritis (RA). Pre-clinical studies on cytokine function and investigations in experimental arthritis models suggest a pathogenic role of GM-CSF in the disease supported by the consistent relationship between macrophage infiltration in the synovium and clinical signs and symptoms of RA. We present the results of a double-blind, placebo-controlled phase 1b/2a (first in patient) study (NCT01023256) in patients with mild to moderate RA. Patients received a 4-wk. active treatment with subsequent follow-up until wk. 16.
Methods:
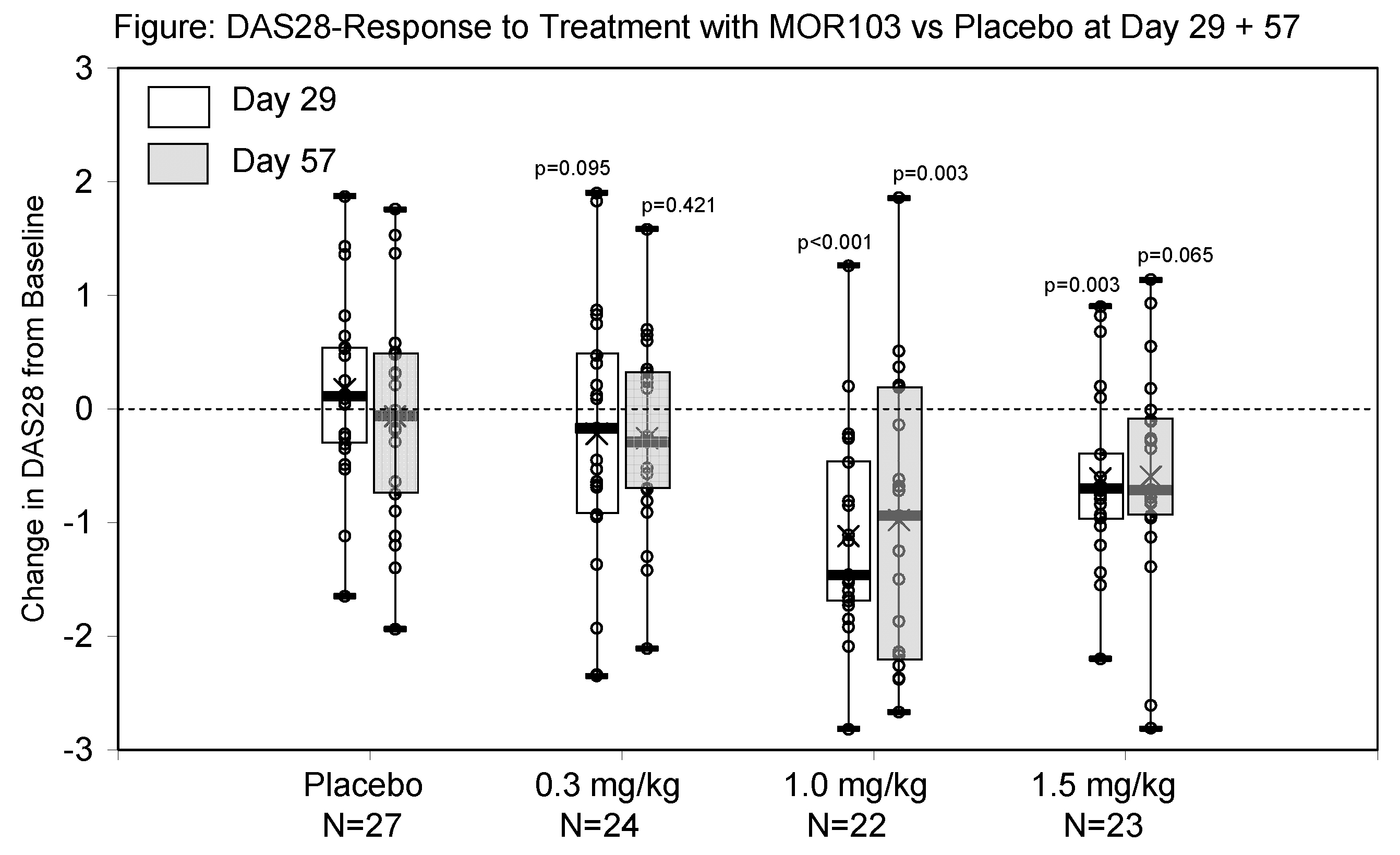
In this sequential ascending dose study safety and clinical activity of MOR103 were evaluated in 96 European patients (MOR103 69; placebo 27) with active RA despite ongoing treatment. Patients with active adult-onset RA of ≥6 mos. duration, and moderate disease activity (≥ 3 swollen and tender joints), elevated CRP, a DAS28-ESR≤5.1, and stable concomitant RA treatment were randomized into i.v. MOR103 (0.3 mg/kg, 1 mg/kg, or 1.5 mg/kg 1qW) or placebo, respectively. Clinical activity was assessed by ACR20 response, DAS28-changes and EULAR responses at Day 29 and 57. In addition the change in RAMRIS scores of sequential MRI scans was assessed as an imaging parameter for the treatment effect on the predominantly affected hand.
Results:
Of the 96 enrolled patients, 85 (placebo 22; MOR103 63) completed the study through 16 wks.: 1 placebo treated subject discontinued due to AE. Overall AEs were mild or moderate, reported with similar frequencies across different cohorts. Most frequent AEs in the MOR103 group were nasopharyngitis and worsening of RA (in all but one patient in post-treatment follow-up). More related AEs were reported in placebo (25.9%) than in active groups (14.5%). SAEs were reported in 1 placebo and 1 MOR103 (0.3 mg/kg) dosed subject. In both cases the clinical event [paronychia (placebo) and pleurisy (MOR103)] had to be classified as serious due to hospitalization. Patients treated with MOR103 1 mg/kg met the endpoint ACR20 in 68.2% compared with 7.4% in placebo by wk. 4 (p=0.0001) and in 30.4% in the 1.5 mg/kg treated cohort. A significant decrease of DAS28 was seen in the M0R103 group with a mean difference of 1.12 and 0.61 at wk. 4 in the 1.0 mg/kg and 1.5 mg/kg cohorts, while a slight increase of 0.17 was documented for placebo (Figure). EULAR response criteria were fulfilled in 68.2% (1.0 mg/kg) and 69.5% (1.5 mg/kg) vs. 7.4% in placebo. The clinical activity was mirrored by a reduction in the RAMRIS score for synovitis of up to -1.5 within 4 wks.
Conclusion:
MOR103 demonstrated rapid and significant clinical activity compared to placebo, most pronounced at the 1.0 mg/kg dosage in this study. Short-term safety and tolerability remained in the range of placebo. These promising results prove the concept of GM-CSF blockade in RA thereby supporting further clinical development of MOR103.
Disclosure:
F. Behrens,
Morphosys,
5;
M. Ostergaard,
Morphosys,
5;
R. Stoilov,
Morphosys,
5;
P. Wiland,
Morphosys,
5;
T. W. Huizinga,
Morphosys,
5;
V. Y. Berenfus,
Morphosys,
5;
P. P. Tak,
Morphosys,
5,
GlaxoSmithKline,
3;
S. Vladeva,
Morphosys,
5;
J. Rech,
Morphosys,
5;
A. Rubbert-Roth,
Morphosys,
5;
M. Korkosz,
Morphosys,
5;
D. Rekalov,
Morphosys,
5;
I. A. Zupanets,
Morphosys,
5;
B. J. Ejbjerg,
Morphosys,
5;
J. Geiseler,
Morphosys,
5;
J. Fresenius,
Morphosys,
5;
R. P. Korolkiewicz,
Morphosys,
3;
A. J. Schottelius,
Morphosys,
3;
H. Burkhardt,
Morphosys,
5.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/first-in-patient-study-of-anti-gm-csf-monoclonal-antibody-mor103-in-active-rheumatoid-arthritis-results-of-a-phase-1b2a-randomized-double-blind-placebo-controlled-trial/