Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: BMS-986165 is an oral, selective inhibitor of TYK2, an enzyme that activates signal transducer and activator of transcription (STAT)-dependent cytokine signaling pathways, including the IL-12/IL-23 signaling pathway. IL-12/IL-23 signaling has a key role in the pathogenesis of skin disease in both psoriasis (PsO) and PsA. In a 12-week Phase 2 study in adults with PsO that included those with musculoskeletal symptoms, BMS-986165 demonstrated a dose-dependent Psoriasis Area and Severity Index (PASI) 75 response and a favorable safety profile.1 Further analysis was conducted in this study to explore the impact of BMS-986165 on pain scores using exposure–response modeling in those with baseline musculoskeletal symptoms.
Methods: A longitudinal exposure–pain visual analog scale (VAS) score model was developed to describe the effect of BMS-986165 on pain VAS scores over time in patients with PsO and musculoskeletal symptoms at baseline in the Phase 2 study (NCT02931838).1 The exposure data (steady-state minimum plasma drug concentration) were derived from a population pharmacokinetics model and the response data were the pain VAS scores measured on Days 1, 8, 15, 29, 57, 85 (on treatment), and 115 (off-treatment follow-up). The response data from 87 patients were modeled with exposure using a nonlinear mixed effects model. Simulations were performed to compare results for different dosing regimens. Additionally, correlation of median pain VAS and PASI scores were compared across the dose groups.
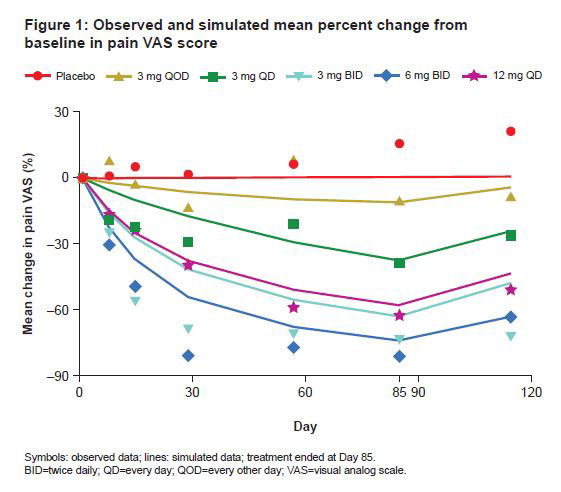
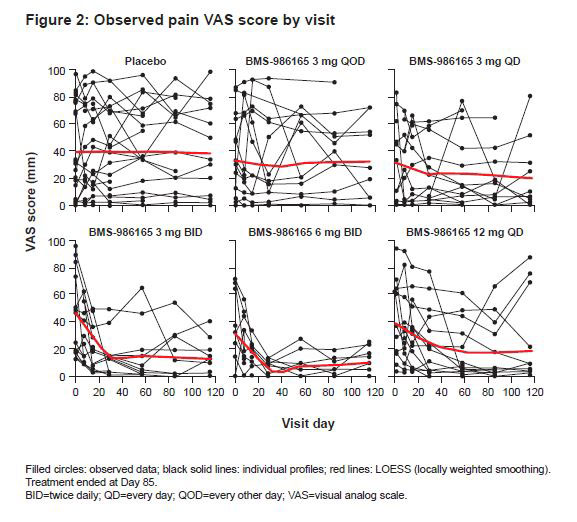
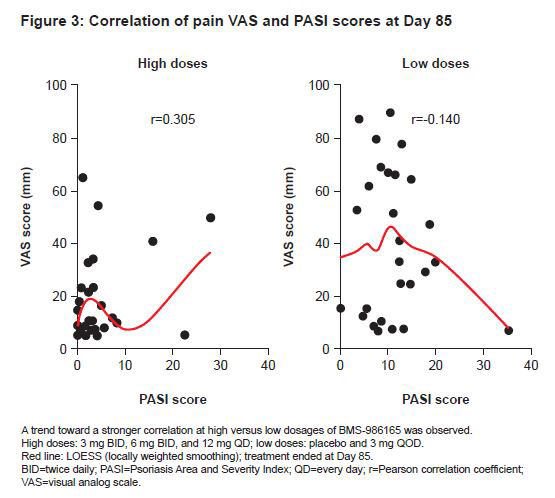
Results: Median model-simulated percent change from baseline pain VAS score ranged from -62% to -89% across BMS-986165 dosage regimens over 12 weeks; observed and simulated mean percent change are shown in Figure 1. Visual predictive checks of the exposure–response model demonstrated the model-predicted VAS score generally agreed overall with the observed data. BMS-986165 demonstrated a dose-related improvement in pain VAS score, with a sustained effect observed after treatment ended on Day 85 (Figures 1 and 2). A clear relationship between BMS-986165 exposure and pain VAS score reduction can be established. Correlation between median pain VAS and PASI scores was weak overall, suggesting that pain improvements could not be entirely explained by improvements in skin disease (Figure 3).
Conclusion: The relationship between BMS-986165 exposure and pain VAS score, in patients with PsO and baseline musculoskeletal symptoms, was captured using a nonlinear mixed effects exposure–response model. BMS-986165 demonstrated dose-dependent improvements in pain. Pain and PASI scores were weakly correlated. The decreased pain scores with BMS-986165 treatment, which were not well-correlated with improvements in PASI scores, were suggestive of improvements in joint inflammation.
Reference:
- Papp K, et al. N Engl J Med. 2018;379:1313-1321.
Writing support: Linda Brown, Caudex, funded by Bristol-Myers Squibb.
To cite this abstract in AMA style:
Shen J, Chimalakonda A, Nowak M, Throup J, Banerjee S, Girgis I. Exposure–Response Modeling of an Oral, Selective Tyrosine Kinase 2 (TYK2) Inhibitor, BMS-986165, for Pain Visual Analog Scale Score, in Patients with Psoriasis and Musculoskeletal Symptoms [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/exposure-response-modeling-of-an-oral-selective-tyrosine-kinase-2-tyk2-inhibitor-bms-986165-for-pain-visual-analog-scale-score-in-patients-with-psoriasis-and-musculoskeletal-symptoms/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/exposure-response-modeling-of-an-oral-selective-tyrosine-kinase-2-tyk2-inhibitor-bms-986165-for-pain-visual-analog-scale-score-in-patients-with-psoriasis-and-musculoskeletal-symptoms/