Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: While the composite ACR core data set is considered a standard for assessing disease severity and improvement in PsA, understanding the response rates of the individual ACR components may be more useful in guiding clinical decisions.1 Here we present the efficacy response across all ACR components to Week 108 in PsA patients treated with ixekizumab (IXE), a high-affinity monoclonal antibody that specifically targets IL-17A, approved for the treatment of adults with active PsA and moderate-to-severe psoriasis.
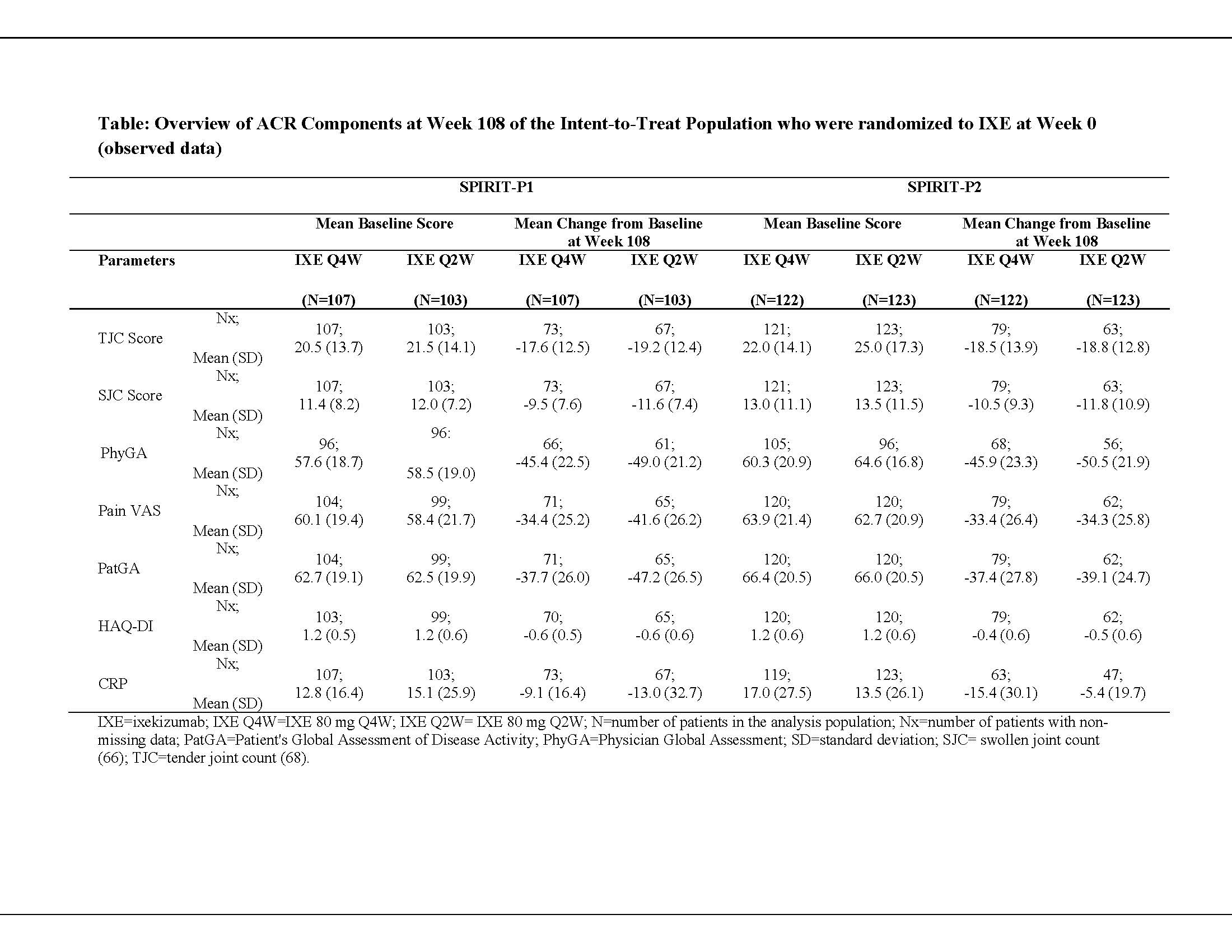
Methods: In 2 Phase 3 randomized controlled trials, patients who met Classification Criteria for PsA and were biologic DMARD (bDMARD)-naïve (SPIRIT-P1) or had an inadequate response to 1 or 2 TNFi (SPIRIT-P2) received subcutaneous IXE 80 mg every 2 weeks (Q2W) or every 4 weeks (Q4W), after a 160-mg starting dose. The change from baseline at Week 108 for all components of ACR was assessed: tender joint count (TJC), swollen joint count (SJC), physician global assessment (PhyGA), patient’s assessment of pain visual analog scale (VAS), patient’s global assessment of disease activity (PatGA), HAQ-Disability Index (HAQ-DI) and CRP. Observed results are reported for the intent-to-treat (ITT) population who were initially randomized to IXE at baseline (Week 0). At Week 16, inadequate responders (< 20% improvement in both TJC and SJC) received rescue therapy and were analyzed as non-responders at Weeks 20 and 24. From Week 32 and at each subsequent visit, patients were discontinued from the study if they failed to achieve ≥20% improvement in both TJC and SJC.
Results: When evaluating group-level changes to 2 years (Week 108), patients treated with the approved PsA dosing of IXE Q4W showed comparable mean change from baseline in clinical components of ACR (TJC, SJC, PhyGA, pain VAS, PatGA, and HAQ-DI) regardless of prior TNFi failure (Table). The median (maximum, minimum) change from baseline in laboratory measure of CRP was comparable between IXE Q4W-treated patients in both populations: SPIRIT-P1, -3.9 (-99.1, 9.2); SPIRIT-P2, -2.3 (-142.0, 8.2).
Conclusion: Irrespective of whether bDMARD-naïve or having prior inadequate response or intolerance to TNFi, IXE Q4W-treated patients with PsA achieved comparable efficacy across all clinical components of ACR as measured by mean change from baseline at Week 108.
Reference: 1. Pincus T. Clin Exp Rheumatol. 2005;23(Suppl 39):S109-13.
To cite this abstract in AMA style:
Turkiewicz A, Trevelin Sprabery A, Gellett A, Young Park S, Constantin A. Ixekizumab Demonstrates Consistent Improvement up to Week 108 in Psoriatic Arthritis Across Individual ACR Components for Patients Naïve to Biologic DMARDs or with Previous Inadequate Response to TNF Inhibitors [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/ixekizumab-demonstrates-consistent-improvement-up-to-week-108-in-psoriatic-arthritis-across-individual-acr-components-for-patients-naive-to-biologic-dmards-or-with-previous-inadequate-response-to-tnf/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ixekizumab-demonstrates-consistent-improvement-up-to-week-108-in-psoriatic-arthritis-across-individual-acr-components-for-patients-naive-to-biologic-dmards-or-with-previous-inadequate-response-to-tnf/