Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Tildrakizumab (TIL) is an anti–interleukin-23p19 monoclonal antibody approved by the FDA for treatment of moderate-to-severe plaque psoriasis1,2 and is under investigation for treatment of PsA. The objective of this analysis was to evaluate the safety of TIL in patients with active PsA at 24 weeks from the randomized, double-blind, placebo-controlled, multiple-dose, phase 2b TIL trial (NCT02980692).
Methods: Patients ≥18 years old with a diagnosis of PsA (by the Classification of Psoriatic Arthritis criteria3) and ≥3 tender and ≥3 swollen joints were randomized 1:1:1:1:1 to 5 treatment arms (administered by subcutaneous injection)—200 mg TIL every 4 weeks (Q4W), 200 mg TIL every 12 weeks (Q12W), 100 mg TIL Q12W, 20 mg TIL Q12W, or placebo to week 24. Treatment-emergent adverse events (TEAEs) were monitored throughout the study; and defined as events that occurred on/after the first dose of TIL but before week 24, or on/before the last dose if the subject discontinued treatment before week 24 (coded by Medical Dictionary of Regulatory Activities v20.1).
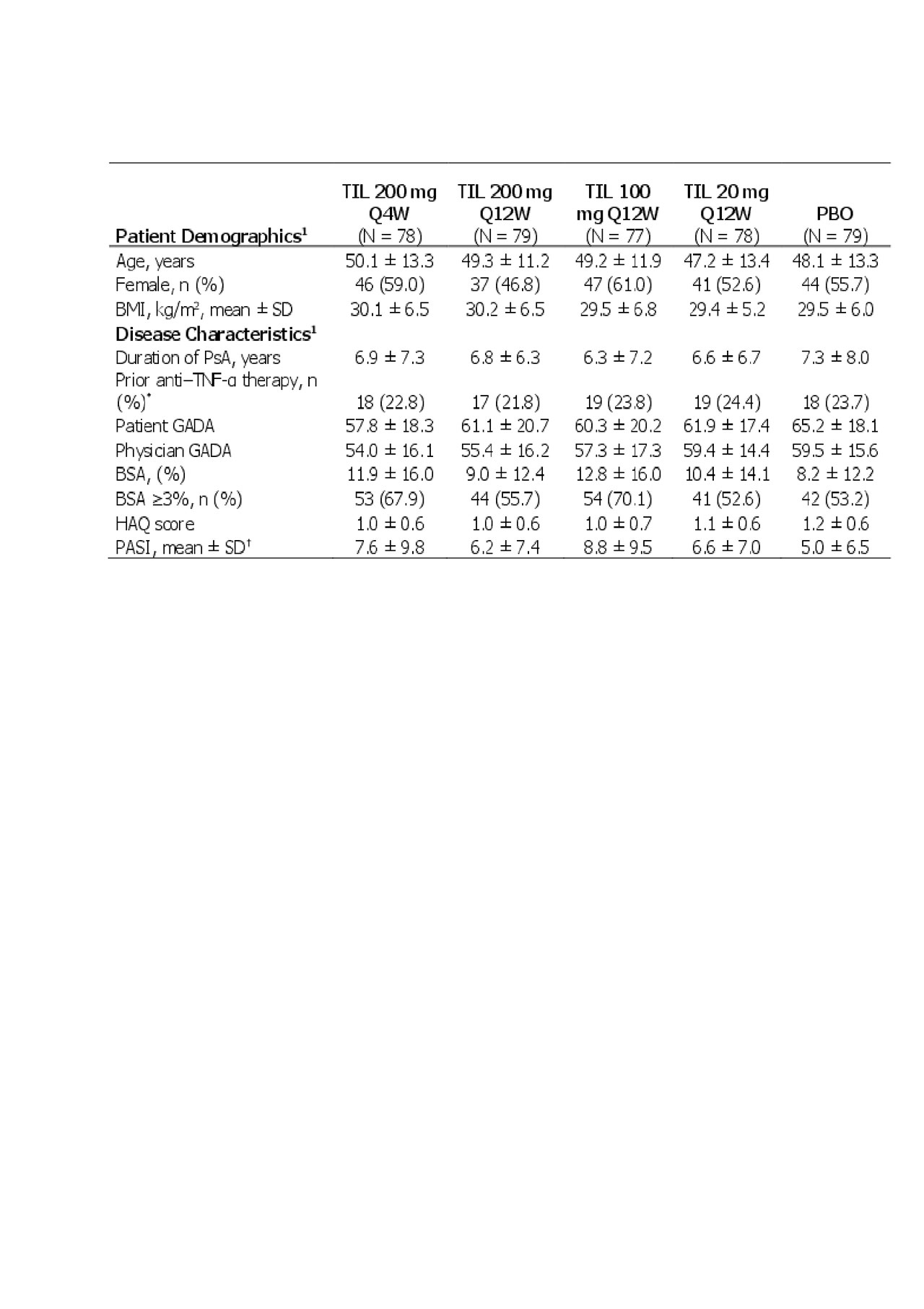
Results: In total, 391/500 patients screened met inclusion criteria. Patient demographics and baseline disease characteristics were consistent across the groups (Table 1). At 24 weeks, 61 (78.2%) TIL 200 mg Q4W, 64 (81.0%) TIL 200 mg Q12W, 60 (77.9%) TIL 100 mg Q12W, 71 (91.0%) TIL 20 mg Q12W, and 74 (93.7%) placebo patients completed treatment, respectively. Reasons for discontinuation included an adverse event in 1 (1.3%), 2 (2.5%), 1 (1.3%), 2 (2.6%), and 0 patients, respectively, and lack of efficacy in 9 (11.5%), 12 (15.2%), 13 (16.9%), 0, and 0 patients, respectively.
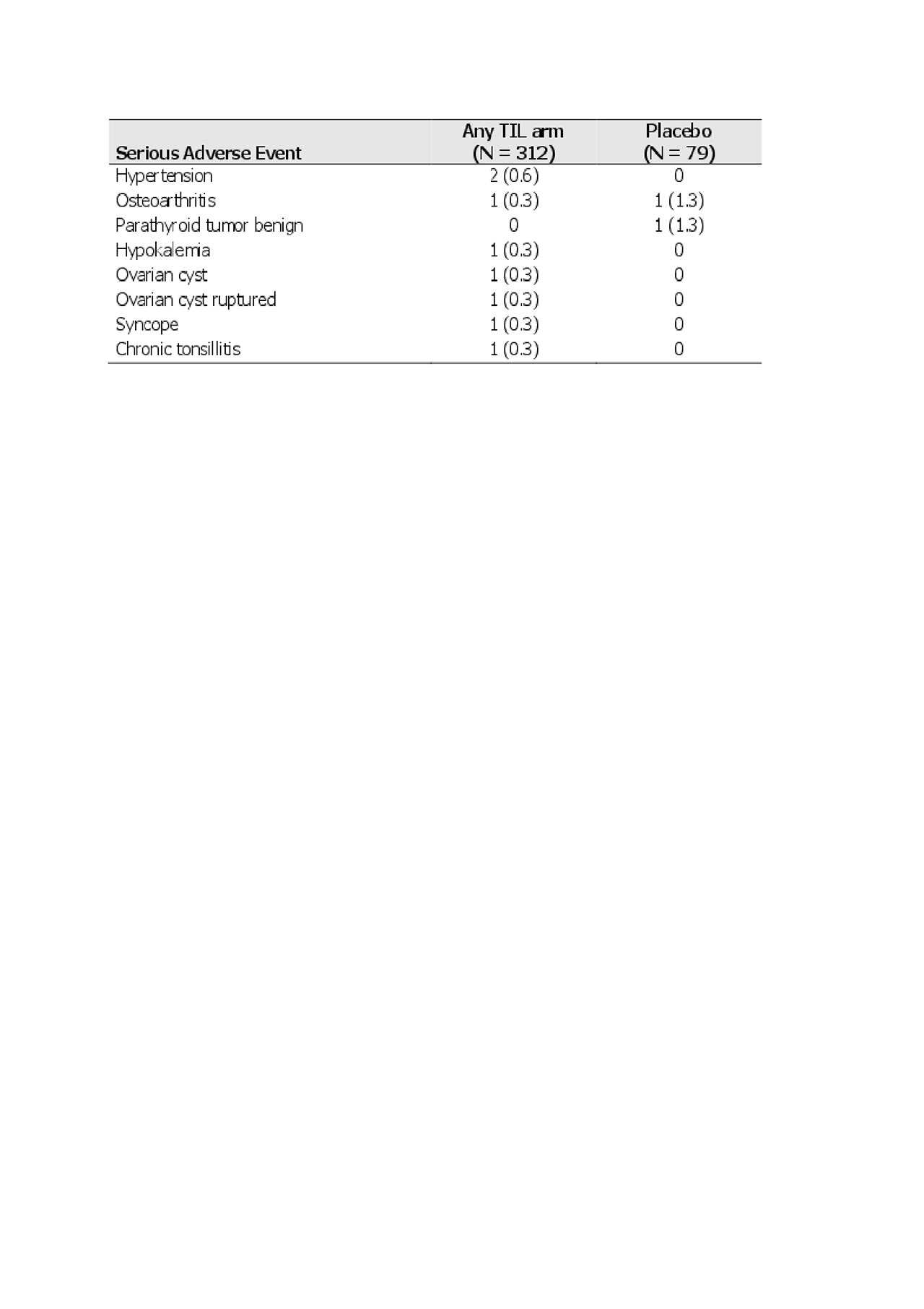
At week 24, 156 (50.0%) TIL-treated and 34 (43.0%) placebo-treated patients had any TEAE, 7 (2.2%) and 2 (2.5%) a serious TEAE, and 35 (11.2%) and 10 (12.7%) a treatment-related TEAE, respectively. The most commonly reported TEAEs were nasopharyngitis (17 [5.4%] TIL-treated vs 5 [6.3%] placebo-treated), headache (15 [4.8%] vs 2 [2.5%]), and hypertension (11 [3.5%] vs 4 [ 5.1%]), respectively (Table 2). The most commonly reported serious TEAE was hypertension (2 [0.6%] in TIL-treated patients, Table 3). One patient (0.3%) in the TIL group had a serious infection (chronic tonsillitis). At 24 weeks, there were no reports of candidiasis, inflammatory bowel disease, major adverse cardiac events, significantly increased liver enzymes, or malignancies. In addition, no deaths or discontinuations due to TEAEs were reported. There were no significant elevations in laboratory parameters that led to a serious TEAE designation.
Conclusion: These findings demonstrate that TIL is well tolerated with low rates of TEAEs and serious TEAEs reported during the first 24 weeks of the phase 2b trial.
Editorial support was provided by Puneet Dang, PhD, of AlphaBioCom, LLC.
References:
- Reich K, et al. Lancet. 2017; 390:276-288.
- ILUMYA™ (tildrakizumab-asmn) injection, for subcutaneous use. [Full prescribing information]; August 2018.
- Taylor W, et al. Arthritis Rheum.2006; 54(8):2665-2673.
Data presented as mean ± SD unless otherwise noted.
1Shown for randomized patients who received ≥1 dose of study drug.
*For prior anti–TNF-α therapy, total patients analyzed -N- = 79, 78, 80, 78, and 76 for TIL 200 mg Q4W, TIL 200 mg Q12W, TIL 100 mg, TIL 20 mg, and PBO.
†For prior baseline PASI, total patients analyzed -N- = 75, 79, 76, 75, and 75 for TIL 200 mg Q4W, TIL 200 mg Q12W, TIL 100 mg, TIL 20 mg, and PBO.
BMI, body mass index; BSA, body surface area; GADA, global assessment of disease activity; HAQ, health assessment questionnaire disability index; PBO, placebo; Q4W, every 4 weeks; Q12W, every 12 weeks; SD, standard deviation; TIL, tildrakizumab.
Shown as n -%- for randomized patients who received ≥1 dose of study drug.
TIL, tildrakizumab.
Shown as n -%- for randomized patients who received ≥1 dose of study drug.
TIL, tildrakizumab.
To cite this abstract in AMA style:
Ritchlin C, Strand V, Ballerini R, Chou R, Rozzo S, Mendelsohn A, Kavanaugh A. Safety of Tildrakizumab in Psoriatic Arthritis: An Interim Analysis from a Randomized, Double-blind, Placebo-controlled Phase 2b Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/safety-of-tildrakizumab-in-psoriatic-arthritis-an-interim-analysis-from-a-randomized-double-blind-placebo-controlled-phase-2b-trial/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-tildrakizumab-in-psoriatic-arthritis-an-interim-analysis-from-a-randomized-double-blind-placebo-controlled-phase-2b-trial/