Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Ustekinumab (UST) is a new biological Disease-modifying AntiRheumatic Drug (bDMARD) available in psoriatic arthritis (PsA), targeting respectively IL12-23. Real-world data are missing for this drug on the contrary to TNF inhibitors. Median drug survival of TNF inhibitors is between 10 and 12 months according to the most recent studies (1).There are only few studies with a low number of patients in UST. Studying drug survival in PsA is important, since new therapies have been launched such IL-17 inhibitors or apremilast.
Methods: This is a retrospective, national, multicentric, non-sponsored by any pharmaceutical company study from patients suffering from PsA (CASPAR criteria or diagnosis confirmed by the rheumatologist) from July 2011 to March 2019. UST has been launched in October 2014. Drug survival was defined as the time from initiation to discontinuation (stop/switch) of bDMARDs. Kaplan-Meier survival curves and Cox-regression analyses [hazard ratios (HR) and 95% confidence intervals (CIs)] were used adjusting for patient demographics, disease characteristics, corticosteroid therapy, co-therapy with methotrexate, previous line of bDMARDs posology of UST and switch to 90 mg every 3 months when patients were at 45 mg every 3 months. Reasons of discontinuation were collected and pooled as primary failure, secondary failure and adverse events. Primary inefficacy was defined as an insufficient response to treatment during the six months after the initiation. Secondary inefficacy was defined as a loss of response to treatment after an initial six-month response.
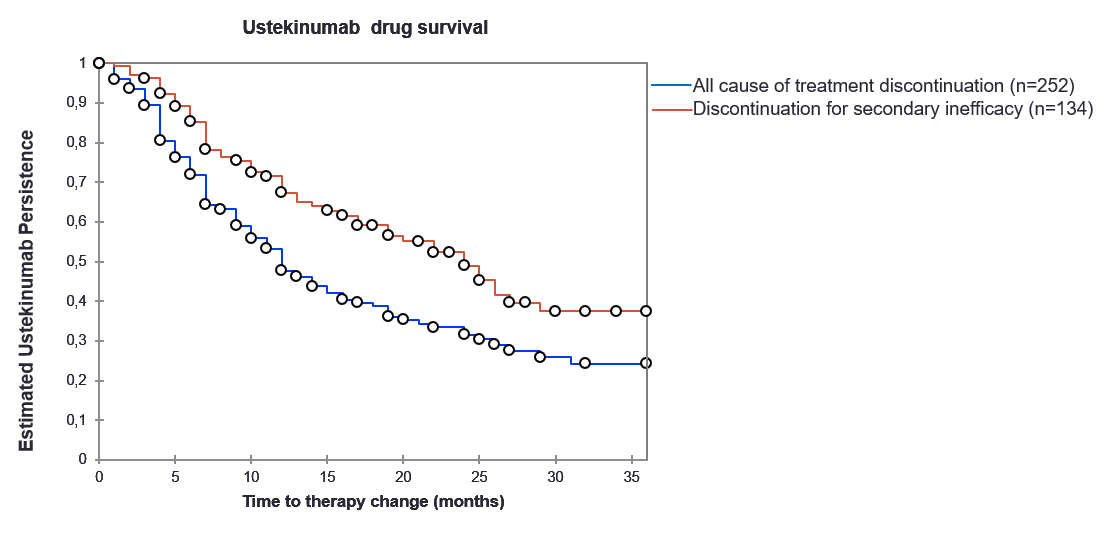
Results: 252 patients were included with a mean follow-up ≥ 6 months: 183 patients satisfy CASPAR. At baseline, the mean age was 49.2 (11.2) years old. 58% of patients were women. 49% of patients were non-smoker. The body mass index was 28.8 (6.3) kg/m². The disease duration was 10.2 (8.9) years. 44% of patients presented axial and peripheral form. 97% of patients presented psoriasis. The mean Charlson Index was 1.3 (1.5). Patients were bDMARDs-naïve in 22%, had a previous bDMARDs in 16% and more than two bDMARDs in 54% and had co-therapy with methotrexate in 39% and 27% of patients received corticosteroid therapy . Patients had a switch to 90 mg every 3 months in 10%. The mean DAS28-CRP was 3.88 (1.3) and the mean CRP was 27.5 (42.7) mg/L. The median drug survival for UST was 12 months (95%CI: 10-15) (Fig1). Baseline high CRP levels and satisfying CASPAR criteria were associated with a favorable drug survival for UST, respectively (HR=1.007, 95%CI 1.002–1.012 and HR=5.56, 95%CI 2.79-11.13), whereas disease duration and nail psoriasis were associated with an unfavorable one, respectively (HR=0.965, 95%CI 0.00–0.99 and HR=0.52, 95%CI 0.00-0.84). 14 patients under UST stopped their treatment due to side effects.
Conclusion: This real-world study for drug survival on PsA is among the studies with the largest number of patients for UST. Drug survival of UST seems similar to the survival of TNF inhibitors (1).
(1) Walsh JA et al. Care Spec Pharm. 2018;24:623-631
To cite this abstract in AMA style:
Letarouilly J, Flachaire B, Labadie C, Sellam J, Richette P, Dieudé P, Claudepierre P, Fautrel B, Houvenagel E, Nguyen C, Guyot M, Segaud N, Maury F, Marguerie L, Deprez X, Salmon J, Baudens G, Gervais E, Chary-Valckenaere I, Lafforgue P, Loeuille D, Richez C, Pham T, Flipo R. Drug Survival of Ustekinumab in Psoriatic Arthritis: A Real-World Multicentric Cohort of 252 Patients [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/drug-survival-of-ustekinumab-in-psoriatic-arthritis-a-real-world-multicentric-cohort-of-252-patients/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/drug-survival-of-ustekinumab-in-psoriatic-arthritis-a-real-world-multicentric-cohort-of-252-patients/