Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: In RA, disease activity correlates with physical function1 and there is a link between joint damage and functional disability2. In many countries, RA patients (pts) with inadequate response (IR) to MTX or other conventional DMARDs (cDMARDs) are not eligible for potentially more effective treatments, such as biologic or targeted synthetic DMARDs (tsDMARDs), unless they have high disease activity (HDA). Thus, managing RA pts with persistent moderate disease activity (MDA) despite cDMARD treatment poses a problem. Baricitinib (BARI) is a tsDMARD approved for the treatment of moderate to severe RA in adults. This post-hoc analysis assessed if RA pts with MDA benefit from improved physical function with BARI treatment to the same extent as pts with HDA
Methods: Pts analysed were from the modified intention-to-treat populations in the two BARI phase 3 studies RA-BEAM3 (MTX-IR) and RA-BUILD4 (cDMARD-IR) with moderate to severe disability (HAQ-Disability Index [HAQ-DI] score ≥1), MDA (Simplified Disease Activity Index [SDAI] score 11.1–26.0) or HDA (SDAI score >26.0) and non-missing SDAI data at baseline. All pts fulfilled ACR criteria for RA. Pts from RA-BEAM received BARI 4 mg + MTX once daily (n=396), adalimumab 40 mg every 2 weeks + MTX (n=270) or placebo (PBO) + MTX (n=390); pts from RA-BUILD received BARI 4 mg (n=189) or 2 mg (n=186) or PBO (n=185). Multivariable linear regression (MLR) models were used to estimate mean HAQ-DI scores at baseline and week (wk) 24 for the treatment arms stratified by baseline disease activity (MDA or HDA SDAI). Age, RA duration, BMI, high sensitivity CRP, baseline SDAI disease activity (MDA or HDA), treatment and treatment-by-baseline SDAI interaction were included as covariates. The MLR model for HAQ-DI at wk 24 was further adjusted by baseline HAQ-DI
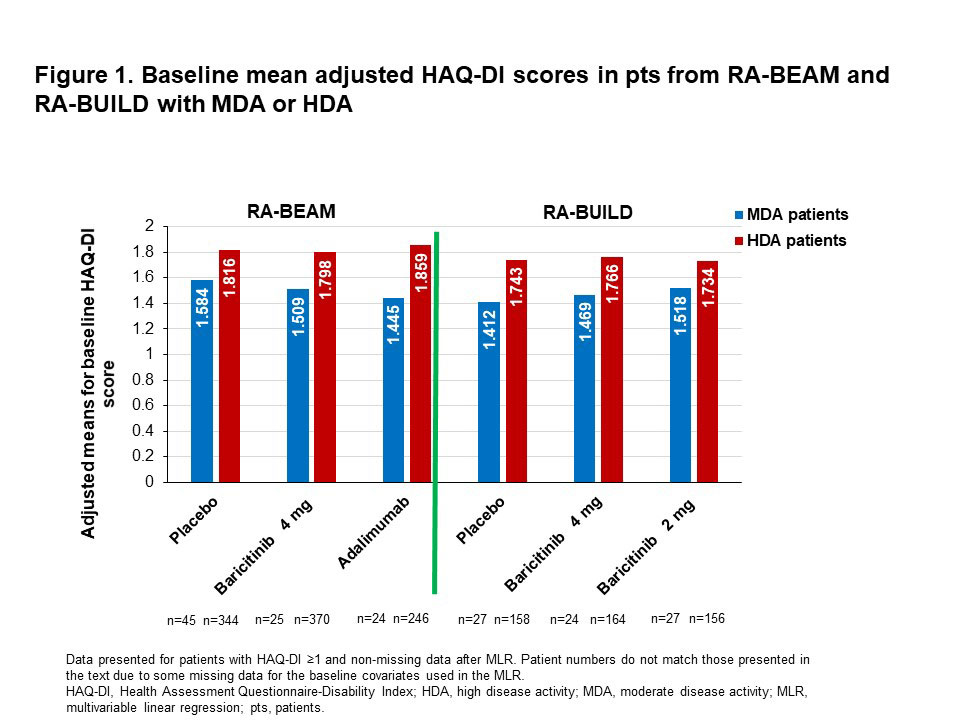
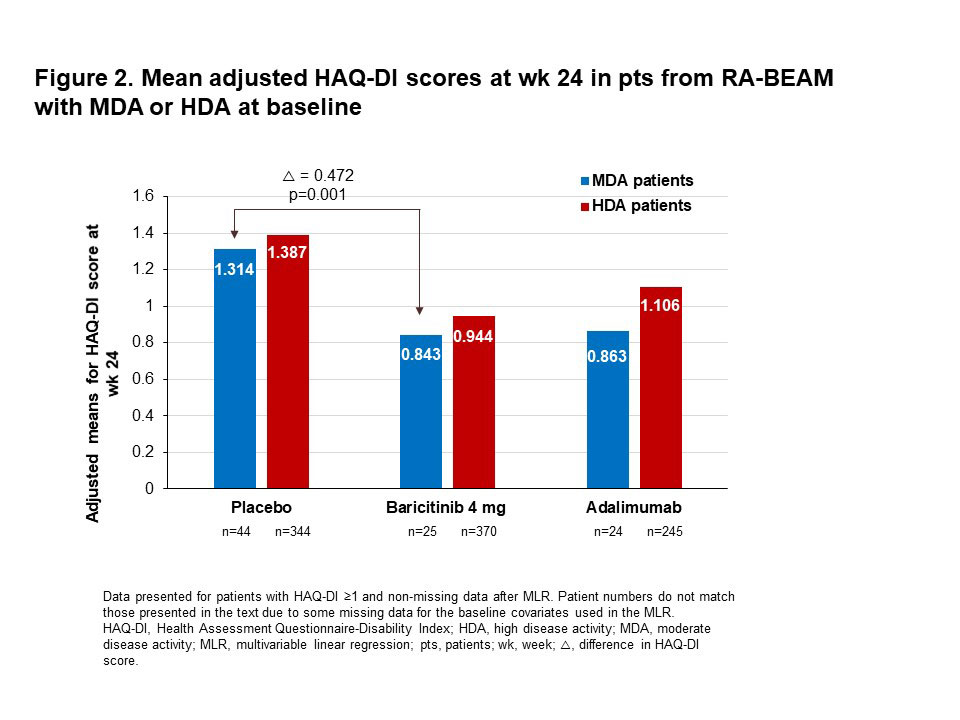
Results: Mean adjusted HAQ-DI scores at baseline were greater in pts with HDA than in those with MDA (Fig. 1). In pts from RA-BEAM with MDA at baseline, the mean adjusted HAQ-DI score at wk 24 was 0.472 points greater in PBO than in BARI 4 mg pts (Fig. 2; p=0.001). A similar pattern of improved physical function with BARI was seen in RA-BUILD, but the adjusted mean difference in HAQ-DI score between PBO and BARI 4 mg (0.263) was not statistically significant (Fig. 3; p=0.109). In pts with HDA at baseline, the mean adjusted HAQ-DI score at wk 24 was 0.443 points greater (p< 0.001) with PBO than with BARI 4 mg in RA-BEAM, and 0.257 points greater (p< 0.001) in RA-BUILD
Conclusion: MTX-IR and/or cDMARD-IR RA pts with MDA and moderate to severe disability at baseline treated with BARI show a similar pattern of improvement in physical function vs. PBO-treated pts to that seen in pts with HDA, supporting early use of BARI in MDA pts. As for those with HDA, pts with persistent MDA despite MTX and/or other cDMARD treatment could benefit from access to biologic and tsDMARDs to prevent disability progression.
- Nikiphorou E et al. Ann Rheum Dis 2016;75:2080
- Kapetanovic MC et al. Arthritis Care Res 2015;67:340
- Taylor P et al. N Engl J Med 2017;376:652
- Dougados M et al. Ann Rheum Dis 2017;76:88
Medical writing support was provided by Sue Chambers and Karen Goa (Rx Communications, Mold, UK), funded by Eli Lilly
To cite this abstract in AMA style:
Kirkham B, Nikiphorou E, López-Romero P, Kouris I, Holzkaemper T, Zaremba-Pechmann L, de la Torre I, Taylor P. Effect of Baricitinib on Functional Impairment in RA Patients with Moderate Disease Activity and an Inadequate Response to Conventional DMARDs [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/effect-of-baricitinib-on-functional-impairment-in-ra-patients-with-moderate-disease-activity-and-an-inadequate-response-to-conventional-dmards/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-baricitinib-on-functional-impairment-in-ra-patients-with-moderate-disease-activity-and-an-inadequate-response-to-conventional-dmards/