Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: ACR guidelines recommend treatment for patients with RA based on baseline (BL) disease activity. In patients with an inadequate response to conventional synthetic DMARDs, escalation to biologic DMARDs is recommended, although specific algorithms are not specified. The aim of this study was to compare treatment response, using disease activity scores, to abatacept (ABA), TNF inhibitors (TNFi) or other non-TNFi targeted (t)DMARDs in patients with RA.
Methods: This was a retrospective, observational cohort study using data from the US-based Rheumatology Informatics System for Effectiveness (RISE) registry. The study period included all observations from inception (June 2014) through December 2017. The index date was the date of first prescription of a new tDMARD. Adult patients (≥18 years) with ≥1 International Classification of Diseases code for RA, a prescription for a new tDMARD, documented results for RF and CCP antibody tests and at least one disease activity score < 6 months before and ≥6 months after index date were included. Covariates were assessed during a 6-month BL period preceding the index date. BL disease activity and changes in disease activity (measured by RAPID3 or CDAI scores) ≥6 months after index date were compared across ABA, TNFi (adalimumab, certolizumab, etanercept, golimumab or infliximab) and other non-TNFi tDMARD (baricitinib, sarilumab, tofacitinib, tocilizumab or rituximab) treatment groups. Disease activity at ≥6 months was compared by treatment and by BL disease activity using validated cut-points (see definitions in footnote below). A one-way ANOVA was used to test patient demographics and the change in RAPID3 and CDAI scores from BL to ≥6 months across treatments.
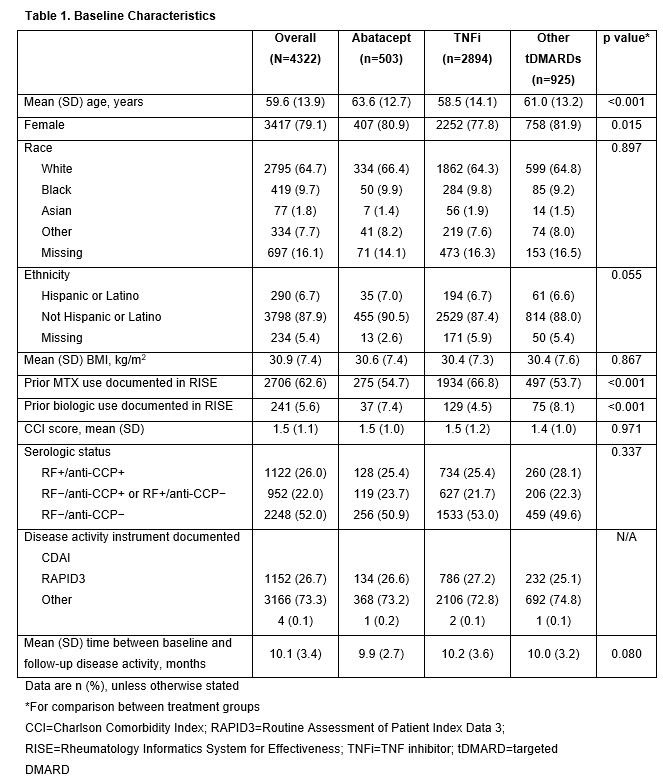
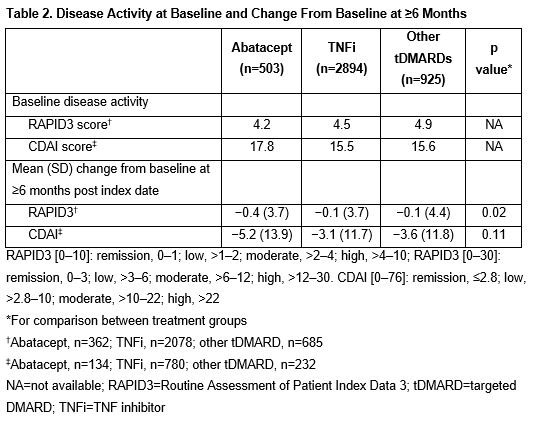
Results: Of the 4322 patients included, 503, 2894 and 925 patients received ABA, TNFi and other tDMARDs, respectively. The mean (SD) age was 59.6 (13.9) years and the majority were female (Table 1). Mean BL RAPID3 scores were similar between groups and mean BL CDAI scores were highest in the ABA group (Table 2). The improvement in RAPID3 score from BL to ≥6 months was greater with ABA than with TNFi or other tDMARDs (p=0.02). Although not statistically significant, a similar trend was seen for change in CDAI score. After 6 months, the proportion of patients with high disease activity was lower in the ABA vs TNFi or other tDMARD groups, across all BL disease activities: 33% of patients in the ABA group improved improved to lower levels of disease activity at ≥6 months after the index date vs patients in the TNFi (17%) or other tDMARD (24%) groups.
Conclusion: In this retrospective study, abatacept-treated patients were older and had higher BL CDAI scores than comparator groups. Nevertheless, after ≥6 months, abatacept was associated with a trend toward greater improvements in disease activity compared with other treatments, regardless of BL disease activity. More work is needed to understand whether these differences are clinically meaningful.
Professional medical writing: Claire Line, PhD, Caudex, funded by Bristol-Myers Squibb.
To cite this abstract in AMA style:
Han X, Bryson J, Crosby D, Evans M, Schmajuk G. Treatment Response to Biologic DMARDs in Patients with RA: A Retrospective Analysis of the RISE Registry [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/treatment-response-to-biologic-dmards-in-patients-with-ra-a-retrospective-analysis-of-the-rise-registry/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-response-to-biologic-dmards-in-patients-with-ra-a-retrospective-analysis-of-the-rise-registry/