Session Information
Date: Monday, November 11, 2019
Title: Epidemiology & Public Health Poster II: Spondyloarthritis & Connective Tissue Disease
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Intravenous (IV) CYC in combination with glucocorticoids is a widely used induction therapy for severe cases of rheumatic diseases such as SLE, SSc, DM and ANCA-associated vasculitis. The dosage of Euro-lupus low dose regimen (six pulses of IV CYC biweekly at a fixed dose of 500 mg), which is one of the standard regimens, does not take account of body size. The fixed dose of 500 mg is equivalent to approximately 300 mg/m2 or less per body surface area (BSA) for average sized European patients, but it can be more than 300 mg/m2 per BSA for patients with smaller physiques. In this study, we evaluated the efficacy and safety of dose adjustment based on patients’ body size in low dose IV CYC treatment for rheumatic diseases.
Methods: In this retrospective cohort study, we analyzed consecutive patients with rheumatic diseases, consisting of SLE, SSc, DM and ANCA-associated vasculitis, who received IV CYC therapy at the rheumatology department of the Kyoto Prefectural University of Medicine in Japan between 2008 and 2019. Patients with a creatinine clearance less than 10 mL/min were excluded. We compared clinical outcomes and adverse events in patients receiving doses above and below 300 mg/m2 for BSA. All data were presented as median [interquartile range (IQR)] or absolute number (percentage). In comparing two groups, we used the Mann–Whitney U test (for continuous variables) or Fisher’s exact test (for categorical variables). The Kaplan–Meier curves were compared using the log rank test. Any p value < .05 was considered statistically significant.
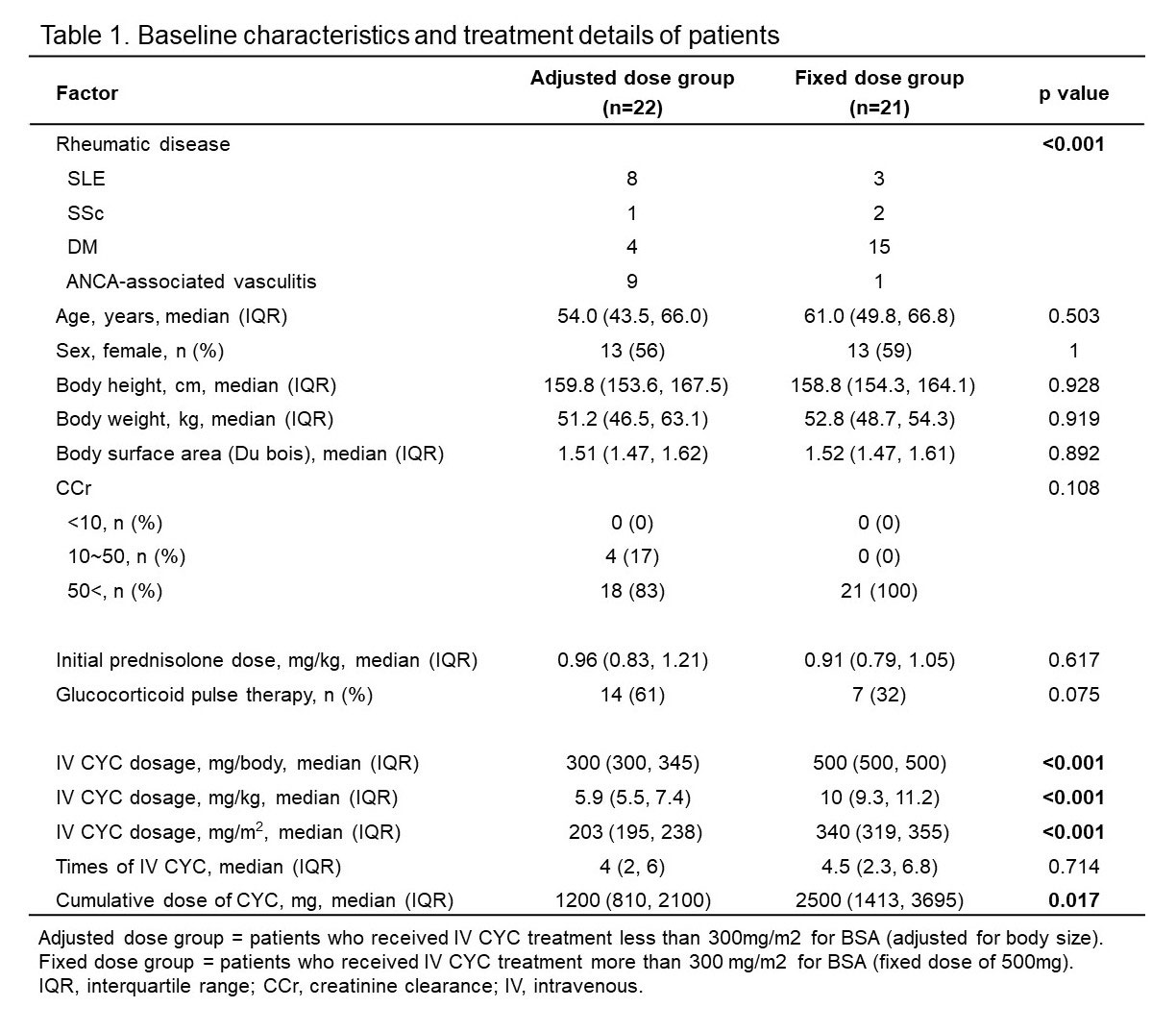
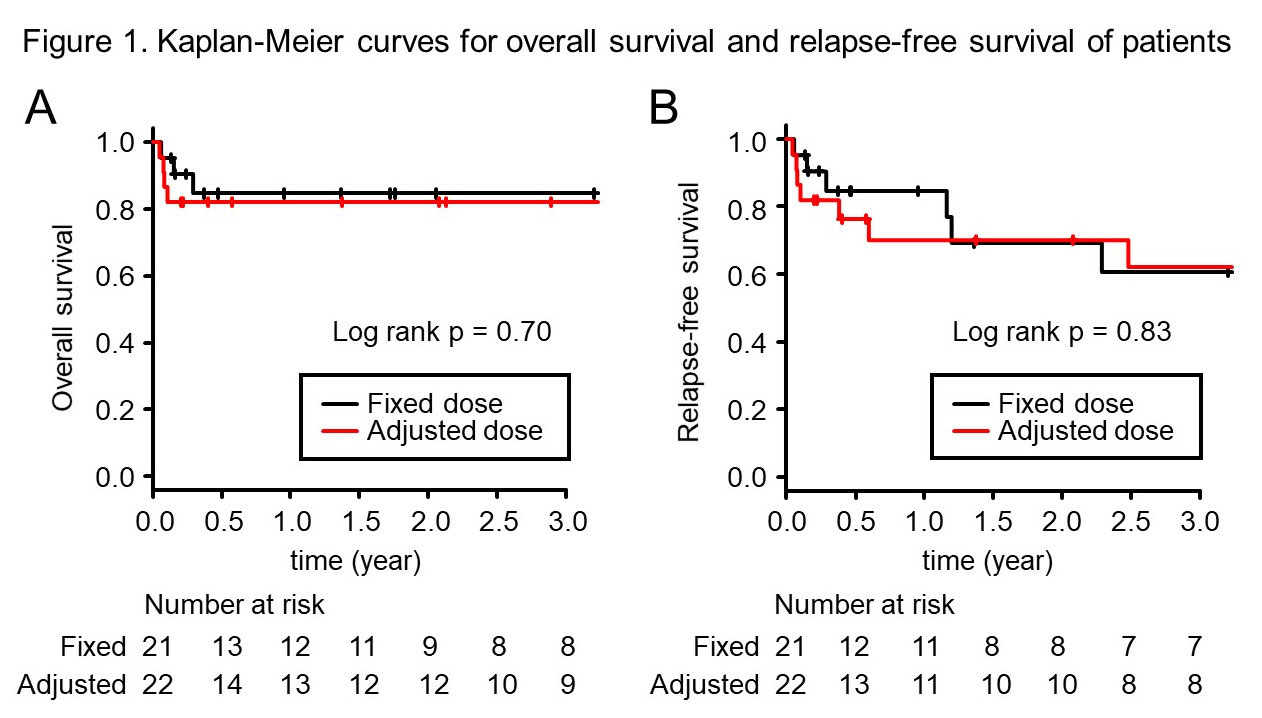
Results: We identified 43 patients (SLE = 11, SSc = 3, DM = 19, ANCA-associated vasculitis = 10), all of whom met the ACR classification criteria. Among them, 22 received IV CYC treatment less than 300mg/m2 for BSA as “adjusted” low dose treatment considering body size, and 21 received more than 300mg/m2 for BSA as “fixed” low dose treatment. There was no difference between the two groups as age, gender, height, weight and body surface area (Table 1). Although there were differences in underlying rheumatic diseases, the dosage of initial prednisolone was similar between the two groups. There was no difference in the frequency of IV CYC administration, but the median dose for IV CYC was lower in the adjusted dose group than in the fixed dose group (300 mg/body vs 500 mg/body, respectively, p < 0.001), and the median cumulative dose was also lower in the adjusted dose group (1200 mg vs 2500 mg, respectively, p = 0.017). Between the two groups, there was no difference in overall survival or relapse-free survival (Figure 1).
Conclusion: Our data indicates that adjusted low dose IV CYC biweekly treatment (less than 300 mg/m2 for BSA) may have equal clinical efficacy and better safety compared to fixed low dose treatment. This dose adjustment of low dose IV CYC treatment based on patients’ body size would be beneficial as an effective and safe remission induction therapy for rheumatic diseases.
To cite this abstract in AMA style:
Hirano A, Kida T, Kasahara A, Sakashita A, Sagawa T, Kaneshita S, Inoue T, Fujioka K, Fujii W, Wada M, Kohno M, Kawahito Y. Efficacy and Safety of Dose Adjustment Based on Patients’ Body Size in Low Dose Intravenous CYC Treatment for Rheumatic Diseases [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-dose-adjustment-based-on-patients-body-size-in-low-dose-intravenous-cyc-treatment-for-rheumatic-diseases/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-dose-adjustment-based-on-patients-body-size-in-low-dose-intravenous-cyc-treatment-for-rheumatic-diseases/