Session Information
Date: Monday, November 11, 2019
Title: Epidemiology & Public Health Poster II: Spondyloarthritis & Connective Tissue Disease
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Many new PsA treatments have emerged without clear guidelines on which therapy to use initially. We describe PsA treatment trends and patient journeys between 2012 and 2018.
Methods: Adult PsA patients were selected if they initiated any therapy (index date) with an oral conventional DMARD (cDMARD; cyclosporine, methotrexate, sulfasalazine, leflunomide, azathioprine, gold, hydroxychloroquine), apremilast (APR), TNF inhibitor (adalimumab, certolizumab, etanercept, golimumab, infliximab), or IL inhibitor (ixekizumab, secukinumab, ustekinumab) from January 1, 2012, to June 30, 2017. Selected patients had ≥12 months pre- and post-index continuous enrollment in the IBM Watson Health MarketScan® Commercial and Medicare Supplemental Database and no history of biologic-indicated autoimmune conditions (ie, diagnosis of rheumatoid arthritis or IBD) or cancer in the pre- or post-index period. Treatment patterns were assessed until end of follow-up. Concluding a line of therapy was defined as discontinuation ( >90-day lapse in therapy) or switch to a new line of PsA therapy (cDMARD, APR, TNF, or IL), whichever occurred first. Patients switching to a new line of therapy or restarting the previous therapy after discontinuation were categorized. Initiating or switching to combination therapy was defined as ≥2 new agents within 14 days, or adding a new agent on current therapy with ≥60 days’ supply overlap. Patient characteristics, first- and second-line PsA agent use, and frequency of treatment sequences were summarized by years and first-line drug categories.
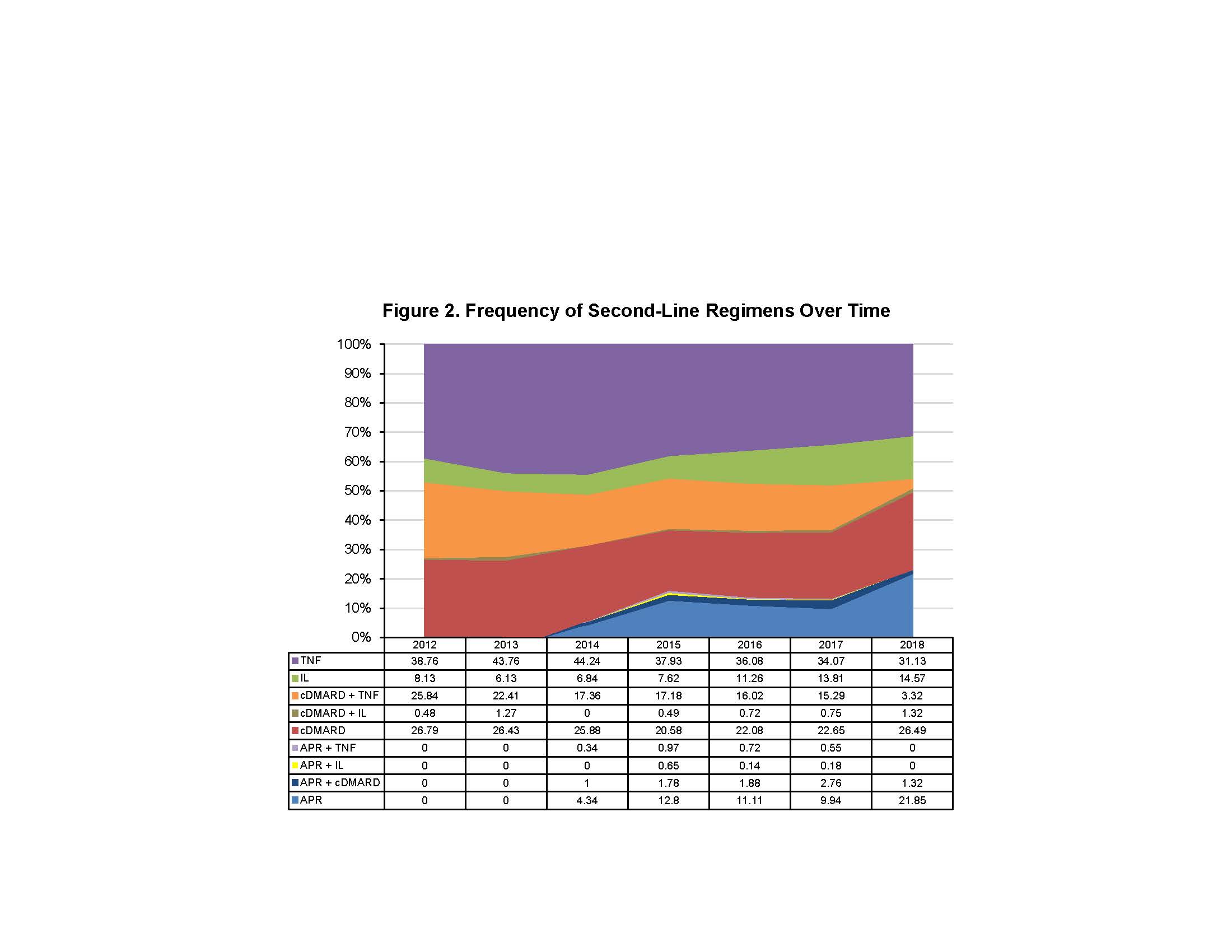
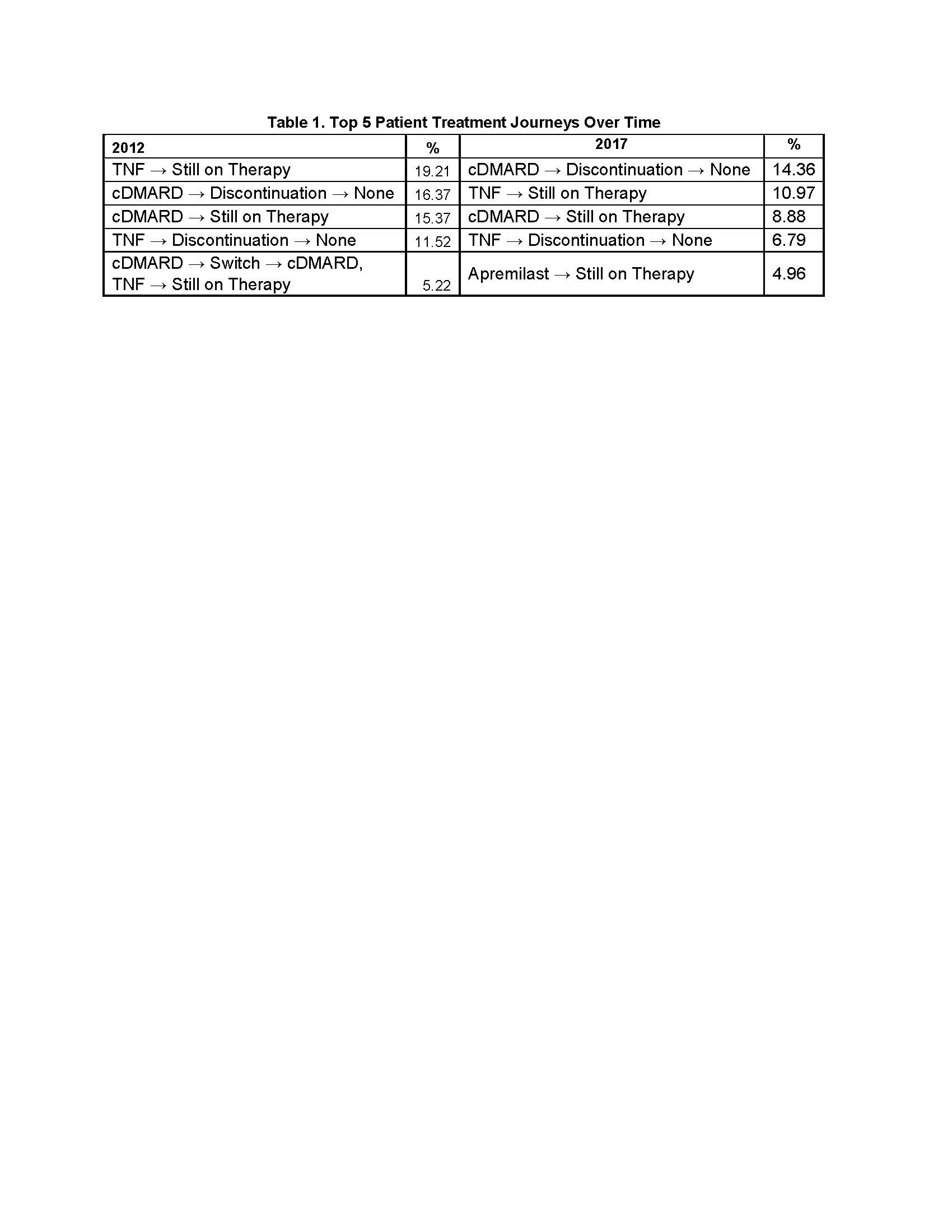
Results: A total of 5,135 PsA patients (cDMARD: n=2,821 APR: n=341, TNF: n=1,756, IL: n=217) were included. Mean age was 48 (SD 11.9) years and 52.3% were female. Mean age was similar among treatment groups, whereas the cDMARD and APR groups had more female patients. Mean length of follow-up was 1,017 (SD 514) days. First-line oral cDMARD and IL use remained consistent from 2012 to 2017. First-line APR use increased over time since its FDA approval in 2014, while TNF use decreased (Figure 1). At end of follow-up, a larger proportion of first-line APR patients remained on therapy (27.9%) vs cDMARD (11.8%), TNF (22.5%), and IL (23.5%). Among those who ended first-line therapy, treatment patterns were as follows: APR had the highest rate of sustainability as staying first line (not progressing to second-line therapy) after discontinuation (30.9%), followed by cDMARD (24.6%), TNF (19.3%), and IL (16.3%). Oral cDMARDs had the highest rate of switching (61.4%), followed by TNF (56.7%), APR (52.0%), and IL (44.0%). IL had the most restart after discontinuation (39.8%), followed by TNF (24.0%), APR (17.1%), and cDMARD (14.0%). Second-line cDMARD use was maintained throughout the study period. Second-line APR and IL use increased while TNF and cDMARD + TNF combination use decreased (Figure 2). Differences in the most frequent 12-month patient treatment journeys were observed during the study period (Table 1).
Conclusion: APR as first- and second-line treatment increased while TNF use decreased during the study period. cDMARD use remained consistent and IL therapy use increased second line. Introduction of newer PsA agents has impacted the treatment paradigm.
To cite this abstract in AMA style:
Husni M, Ung B, Richter S, Pelletier C, Tian M. Assessing Psoriatic Arthritis Treatment Trends and Patient Journeys Between 2012 and 2018 [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/assessing-psoriatic-arthritis-treatment-trends-and-patient-journeys-between-2012-and-2018/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessing-psoriatic-arthritis-treatment-trends-and-patient-journeys-between-2012-and-2018/