Session Information
Date: Sunday, November 10, 2019
Title: 3S107: Pediatric Rheumatology – Clinical I: Systemic JIA (915–920)
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Macrophage activation syndrome (MAS) is the most severe complication of systemic juvenile idiopathic arthritis (sJIA) and
its adult equivalent, adult-onset Still’s disease (AOSD). Because MAS can follow a rapidly fatal course, its prompt recognition and immediate therapeutic intervention are critical. An international collaborative effort has recently led to the publication of the 2016 classification criteria for MAS in sJIA. However these criteria are intended to serve for classification purposes and not as diagnostic tool. The aim of this study was to develop and validate a diagnostic score for timely detection of MAS in patients with sJIA
Methods: The clinical and laboratory features of 362 patients with sJIA-associated MAS and 404 patients with active sJIA without evidence of MAS were collected in a multinational collaborative project. Eighty percent of the study population was used to develop the score and the remaining 20% constituted the validation sample. A Bayesian Model Averaging approach was used to assess the role of each clinical and laboratory variable in the diagnosis of MAS and to obtain the coefficients of selected variables. Variables with an inclusion probability greater than 0.80 composed the final score, named MAS/sJIA (MS) score, which resulted from the linear combination of the values of each variable multiplied by their coefficients. The cutoff that best discriminated MAS from active sJIA was calculated by means of receiver operating characteristic (ROC) curve analysis. Score performance was evaluated in both developmental and validation samples
Results:
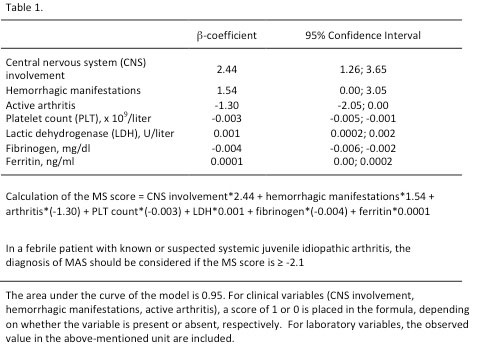
The 7 variables included in the MS score (central nervous system dysfunction, hemorrhagic manifestations, active arthritis, platelet count, fibrinogen, lactate dehydrogenase and ferritin) are presented in Table 1 together with their coefficients and with the MS score calculation formula. The final score ranges from -8.4 to 41.8. A cut-off value > -2.1 revealed the best performance in discriminating MAS from active sJIA, with a sensitivity (SE) of 0.85, a specificity (SP) of 0.95, area under the curve of 0.95 and a kappa value of 0.80. The good performance of the MS score was confirmed in the validation sample (SE 0.89, SP 0.99, AUC 0.97, kappa 0.87)
Conclusion: The MS score is a powerful and feasible tool that may assist practitioners inmaking a timely diagnosis of MAS in patients with sJIA. Future assessments at the bedside could be enhanced and made easier by developing a phone/web application. Considering that sJIA and AOSD are nowadays thought to be part of the same disease spectrum, the MS score might be also useful in timely recognition of MAS in patients with AOSD. The MS score deserves validation in a prospective cohort of patients with sJIA-associated MAS
To cite this abstract in AMA style:
Minoia F, Bovis F, Davì S, Horne A, Fischbach M, Frosch M, Huber A, Jelusic M, Sawhney S, McCurdy D, Silva C, Rigante D, Unsal E, Ruperto N, Martini A, Cron R, Ravelli A. Development and Initial Validation of the MS Score for Diagnosis of Macrophage Activation Syndrome in Systemic Juvenile Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/development-and-initial-validation-of-the-ms-score-for-diagnosis-of-macrophage-activation-syndrome-in-systemic-juvenile-idiopathic-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-and-initial-validation-of-the-ms-score-for-diagnosis-of-macrophage-activation-syndrome-in-systemic-juvenile-idiopathic-arthritis/