Session Information
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Chronic knee pain from osteoarthritis (OA) is frequent in the older adult population. Prior trials have had conflicting results concerning vitamin D’s therapeutic effects, and no large trials have investigated the potential benefits of marine omega-3 fatty acids (n-3 FA) for chronic knee pain. We investigated the effects of vitamin D and n-3 FA supplementation on knee pain in a large clinical trial of older adults.
Methods: The double-blind, placebo-controlled VITamin D and OmegA-3 TriaL (VITAL) randomized 25,871 U.S. adults (women ≥ age 55, men ≥ age 50) in a 2-by-2 factorial design to supplementation with vitamin D (2000 IU/day) and/or n-3 FA (1 gm/day). Prior to randomization, we identified a subgroup of 1430 eligible participants reporting frequent, chronic knee pain and provided additional annual knee pain questionnaires. Analyses included 1398 participants who returned ≥ 1 questionnaire. Questionnaires assessed self-reported pain and functional status with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) at baseline and annually over 5-years of follow-up. For the primary intention-to-treat analysis, we used a repeated measures model to assess the effect of treatment on measures of WOMAC over follow-up, after adjustment for age, sex, and the other treatment arm. Analyses were censored for total knee replacement (TKR). The time by treatment interaction term was used to assess change in WOMAC between randomized groups. In secondary analyses, differences in rates of TKR between treatment groups and placebo were assessed using Cox regression. Reduction in use of daily pain medications (non-steroidal anti-inflammatory and stronger pain medications such as opiates) comparing treatment groups and placebo was ascertained using chi square analysis.
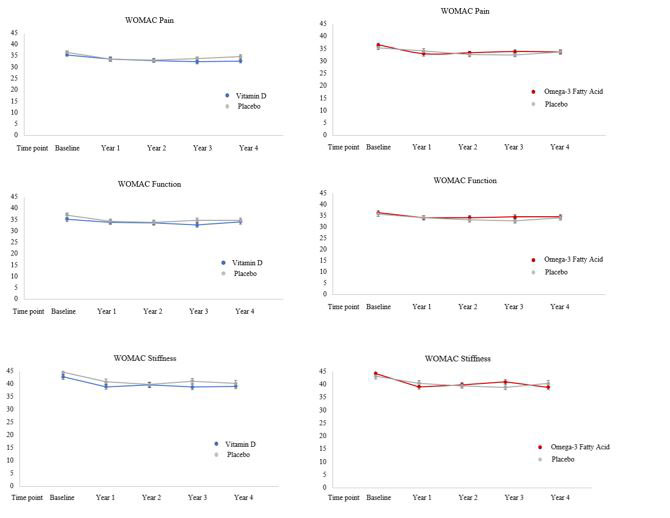
Results: Participants’ mean age was 68 years (SD= 7) and 66% were female. Baseline characteristics were well balanced. In a validation study, 400 participants were randomly selected for record review; of the 226 records received, 92% were confirmed to have knee OA. Over 5-years of follow-up, WOMAC Pain did not differ between vitamin D and placebo or n-3 FA and placebo at any time point. The time by treatment interactions were not statistically significant for either treatment arm (p interaction vitamin D = 0.41, p interaction n-3 FA = 0.77). Similarly, we did not observe clinically or statistically significant differences in WOMAC Function and Stiffness between randomized groups at any time point or over time. (Figure 1) TKR was reported by 140 participants in the vitamin D arm and 156 in placebo; 146 reported TKR in the n-3 FA and 150 in placebo. The hazard ratios for incident TKR in the vitamin D and n-3 FA arms were 0. 97 (95% CI 0.77, 1.22) and 0.99 (95% CI 0.79, 1.24) respectively in comparison to placebo after adjustment for age and sex. Among the daily pain medication users at baseline, there were no significant differences in frequency of pain medication use at last available follow-up between vitamin D and placebo and n-3 FA and placebo.
Conclusion: Neither Vitamin D nor n-3 FA supplementation for up to 5 years improved knee pain, stiffness or function, rate of TKR, or pain medication use in older adults with frequent, chronic knee pain.
To cite this abstract in AMA style:
MacFarlane L, Cook N, Kim E, Lee I, Iversen M, Buring J, Katz J, Manson J, Costenbader K. The Effects of Vitamin D and Marine Omega-3 Fatty Acid Supplementation on Chronic Knee Pain in Older U.S. Adults [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/the-effects-of-vitamin-d-and-marine-omega-3-fatty-acid-supplementation-on-chronic-knee-pain-in-older-u-s-adults/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-effects-of-vitamin-d-and-marine-omega-3-fatty-acid-supplementation-on-chronic-knee-pain-in-older-u-s-adults/