Session Information
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Opioids have long been prescribed for chronic pain conditions, including osteoarthritis (OA). Although the safety of opioids has been questioned, there is little information about their temporal efficacy. Additionally, differences in overall efficacy and safety between strong versus weak opioid classes have not been clearly delineated. We conducted meta-analyses of pain and function at 2, 4, 8, and 12 weeks and analyzed relevant safety outcomes for all opioids, as well as strong versus weak opioids.
Methods: We searched MEDLINE and the Cochrane Database from inception to April 2019 and actively sought unpublished data. Placebo-controlled RCTs assessing the efficacy and/or safety of FDA-approved opioids in patients with knee and/or hip OA were included. We excluded studies using enriched enrollment or double-dummy design involving non-oral treatments. Screening and data extraction were undertaken by two reviewers. We calculated standardized mean differences and risk ratios with 95% confidence intervals. Study quality was assessed using Cochrane risk of bias tool. Random effects meta-analyses were performed, and heterogeneity assessed using the I2 statistic. Subgroup analyses of strong and weak opioids were conducted for pain at every time point, and for safety outcomes. Meta-regression was performed to assess the impact of dosage (morphine equivalency) on pain relief.
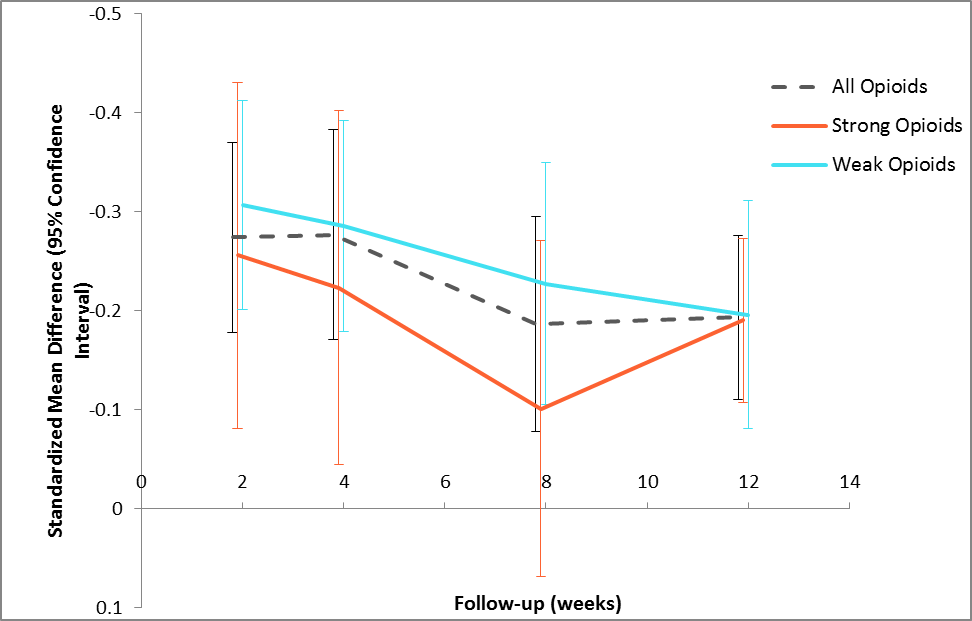
Results: The included 23 RCTs comprised 11,402 participants. 64% were female. The mean age ranged from 54 to 67 years; mean BMI from 28 to 34 kg/m2. All trials were of moderate quality with potential attrition bias being the primary methodological concern. Overall, opioids demonstrated small, statistically significant benefits on pain at each time point ranging from -0.28 to -0.19 (Figure 1); similarly small, statistically significant effects were observed with respect to function at 2, 4, and 12 weeks ranging from -0.26 to -0.16. Opioids had no impact on quality of life or depression; participants who received opioids reported significantly higher quality of sleep. We found that strong opioids had consistently smaller benefits on pain than weak opioids (Figure 1). Meta-regression revealed that opioid dosage (morphine equivalency) has no impact on pain relief (p=0.09) (Figure 2). Methodological bias (selection, attrition) may contribute to the poor performance of strong opioids, or perhaps a pharmacodynamic classification of “strong” has been incorrectly equated with the clinical efficacy of these drugs. Strong opioids showed a safety profile consistently worse than weak opioids, particularly with respect to drug withdrawal symptoms (2.78 [1.41, 5.49] versus 1.06 [0.19, 5.80]) and discontinuations due to adverse events (5.47 [4.63, 6.47] versus 2.77 [2.26, 3.39]).
Conclusion: Overall, opioids demonstrate only small benefits on pain and function from 2 to 12 weeks of treatment and contribute no measurable benefit to QOL or depression versus placebo. Strong opioids demonstrated consistently worse pain relief, with greater risk of any safety outcome than weak opioids. In light of this evidence, clinicians and policy makers should reconsider the utility of strong opioids in the management of OA.
To cite this abstract in AMA style:
Osani M, Lohmander S, Bannuru R. Is There Any Role for Opioids in the Management of OA? [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/is-there-any-role-for-opioids-in-the-management-of-oa/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/is-there-any-role-for-opioids-in-the-management-of-oa/