Session Information
Date: Sunday, November 10, 2019
Title: 3S102: Metabolic & Crystal Arthropathies I: Clinical (898–902)
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: An effect of serum urate on vascular endothelium has been postulated as a mechanism for its association with hypertension and cardiovascular disease. Prior studies in this area have produced conflicting results. We sought to test the hypothesis that serum urate reduction with allopurinol would lead to improvements in endothelial function in young adults with pre-hypertension.
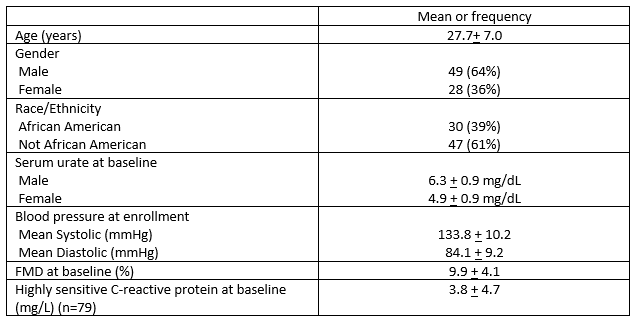
Methods: Single center, double-blinded, crossover trial in which participants were randomly assigned to allopurinol (300 daily mg) and placebo for a period of 4 weeks, separated by a 2-4 week washout. Adults ages 18-40 with baseline systolic blood pressure (SBP) ≥ 120 and < 160 mm Hg or diastolic blood pressure ≥ 80 and < 100 mm Hg, and serum urate ≥ 5.0 mg/dL for men or ≥ 4.0 mg/dL for women were enrolled. Key exclusion criteria included chronic kidney disease, gout, or use of urate-lowering therapies. Endothelial function was assessed with flow-mediated dilation (FMD) testing of the brachial artery using high-resolution ultrasound at four study time points: 1) first baseline, 2) post-allopurinol or placebo treatment, 3) second baseline post-washout, and 4) post-placebo or allopurinol treatment. High-sensitive C-reactive protein (hs-CRP) was measured at the same study points as FMD. Safety assessments were conducted as part of the study.
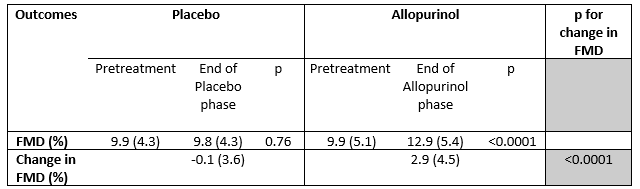
Results: Of the 99 randomized main study participants, 77 completed all FMD testing (Table 1). Among these 77 participants, serum urate decreased by 1.40 + 1.19 mg/dL during the allopurinol period (p< 0.0001) and by a (non-significant) 0.06 + 0.76 mg/dL while taking placebo. The percent change in FMD was highly significant (p< 0.001) during the period assigned to allopurinol (2.9 ± 4.5) versus during the period assigned to placebo (-0.1 ± 3.6)(Table 2 and Figure). Changes in serum urate and FMD were highly correlated (Pearson r = -0.31, p =0.005). Improvements in FMD measurements while taking allopurinol were seen across all studied participant subgroups (younger and older, men and women, African-American and non-African-American, higher categories of serum urate, higher blood pressure at baseline visits). There were no changes from baseline in hs-CRP levels after the allopurinol or placebo phases. No allopurinol hypersensitivity events or other serious adverse events were observed.
Conclusion: Urate-lowering therapy with allopurinol in young adults led to significant improvements in endothelial function when compared with placebo while there was no observed effect on hs-CRP levels.
To cite this abstract in AMA style:
Gaffo A, Calhoun D, Rahn E, Oparil S, Li P, Dudenbostel T, Redden D, Mudano A, Foster J, Feig D, Biggers S, Saag K. Serum Urate Lowering with Allopurinol Improves Endothelial Function in Young Adults [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/serum-urate-lowering-with-allopurinol-improves-endothelial-function-in-young-adults/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/serum-urate-lowering-with-allopurinol-improves-endothelial-function-in-young-adults/