Session Information
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Hydroxychloroquine (HCQ) is near-universally recommended for patients with SLE and is often used in the treatment of RA. Use of HCQ has been associated with reductions in hyperlipidemia, hyperglycemia, and hypercoagulability, which are established risk factors for cardiovascular-metabolic endpoints. We aimed to determine the potential temporal association between HCQ use and cardiovascular (CV) events among patients with SLE or RA.
Methods: We conducted a nested case-control study within a combined inception cohort of SLE and RA using an administrative health database including the entire population in the province of British Columbia, Canada. We identified cases with incident CV events, including non-fatal or fatal myocardial infarction (MI), stroke/transient ischemic attack (TIA), or venous thromboembolism (VTE) (e.g., deep vein thrombosis and pulmonary embolism), as defined by ICD codes. For each case, we identified up to three controls with SLE or RA matched on age, sex, and disease. HCQ exposure was categorized by the time between the last HCQ prescription date covered and the index date as remote ( >365 days), recent (30-365 days) current (< 30 days), or never used.We used conditional logistic regression to assess the association between current use of HCQ or recent discontinuation of HCQ and the risks of incident combined CV events, MI, stroke/TIA, and VTE relative to remote HCQ use. Fully adjusted models included age, sex, SLE vs. RA, Charlson comorbidity index, chronic kidney disease, glucocorticoids, other DMARDs, cardiovascular medication use, and healthcare utilization assessed at the time of SLE or RA diagnosis.
Results: We identified 532 SLE cases matched with 1,249 SLE controls and 9,736 RA cases matched with 28,720 RA controls. The mean age at index date was 74 years. The majority were female (64% of cases and controls) (Table 1). Adjusted odd ratios (ORs) for combined CV events relative to the remote users were 0.83 (95% CI: 0.73, 0.93) for current users and 1.08 (95% CI: 0.91, 1.29) for subjects who recently discontinued HCQ (Table 2). HCQ non-users had the same risk of combined CV events as remote users (OR 0.99 [95% CI: 0.90, 1.08]).
Conclusion: In this nested case-control study within an incident SLE/RA cohort, we found a 17% reduced risk of incident CV events overall associated with current HCQ use. We also identified trends towards reduced risks of MI, Stroke/TIA, and VTE associated with current HCQ use. By leveraging remote users as the comparison group, we reduced the potential for confounding by indication. These findings suggest a preventative benefit of HCQ use in reducing CV disease among patients with SLE and RA.
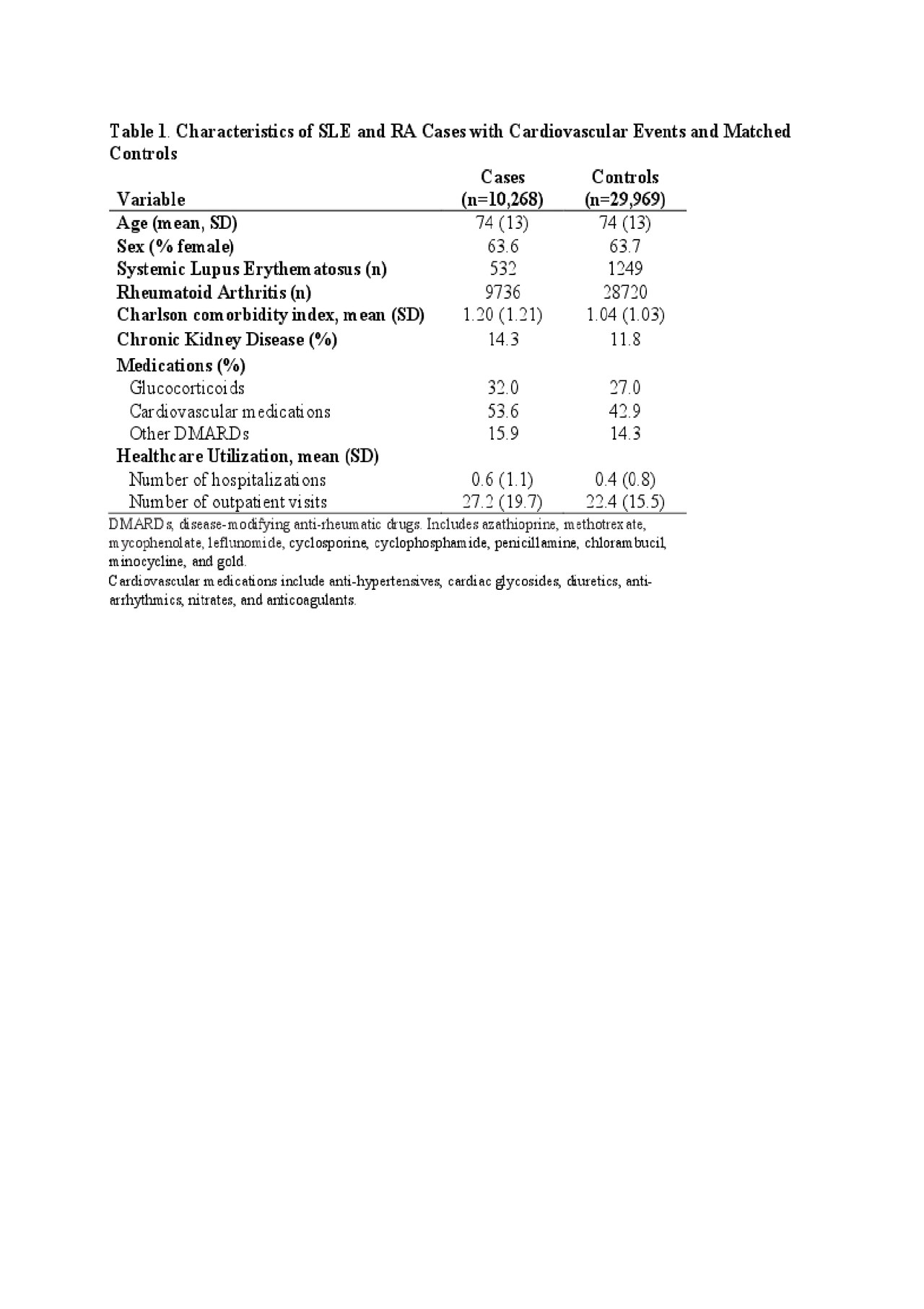
Table 1. HCQ and CVD ACR abstract
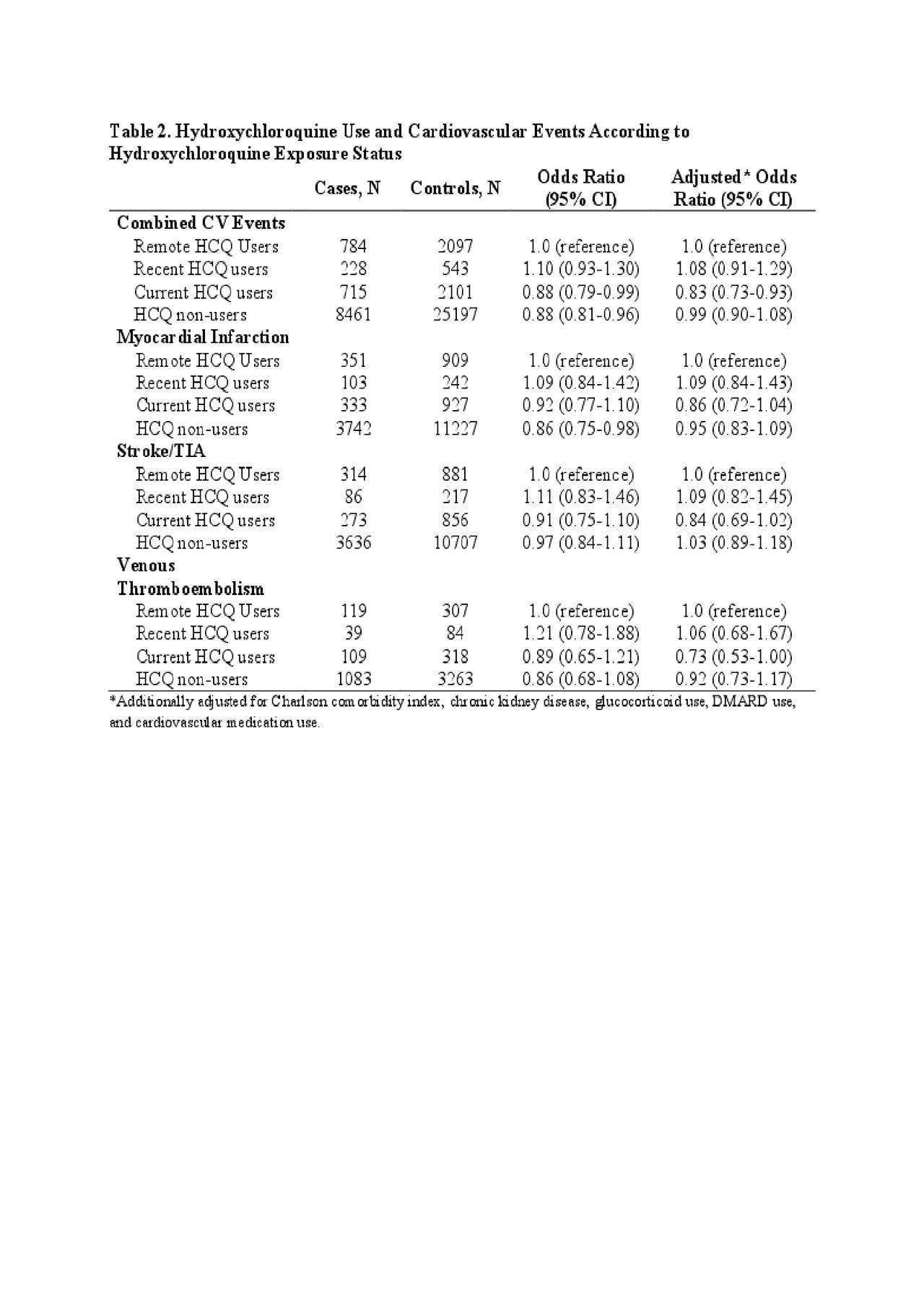
Table 2. HCQ and CVD ACR abstract
To cite this abstract in AMA style:
Jorge A, Lu N, Choi H, Esdaile J, Lacaille D, Avina-Zubieta J. Hydroxychloroquine Use and Cardiovascular Events Among Patients with Systemic Lupus Erythematosus and Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/hydroxychloroquine-use-and-cardiovascular-events-among-patients-with-systemic-lupus-erythematosus-and-rheumatoid-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/hydroxychloroquine-use-and-cardiovascular-events-among-patients-with-systemic-lupus-erythematosus-and-rheumatoid-arthritis/
