Session Information
Session Type: Abstract Submissions (ARHP)
Background/Purpose : The EUMUSC.net project facilitates cooperation between EU Member States and promotes a comprehensive European strategy to optimise musculoskeletal health.
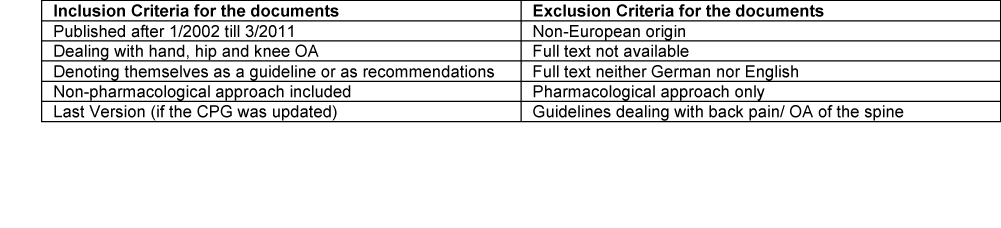
Part of the EUMUSC.net project was devoted to retrieving clinical practice guidelines (CPG) for Osteoarthritis (OA) and to appraise them critically. The purpose of this study was to identify the relevance given to occupational therapy interventions.
Methods: National rheumatological scientific societies, social leagues and health professional associations were contacted and asked to provide relevant documents. Furthermore, a systematic review of the respective literature was conducted in Medline, CINAHL and the Internet. Documents fulfilling pre-defined inclusion and exclusion criteria have been critically appraised by two independent assessors using the AGREE II Instrument. The appraised documents were examined for occupational therapy interventions/occupational therapy (OT).
Results: Six guidelines/recommendations have been included into this study and appraised using the AGREE II Instrument. All six CPGs obtained the highest scores in the domain “Scope and Purpose” followed by remarkably high scores in the domain “Rigor of Development”.
Four CPGs had low scores in the domain “Stakeholder Involvement” and five in the domain “Applicability”. We identified ten interventions relevant to occupational therapy: adapting the environment, assistive devices for activities of daily living, hand exercise, joint protection, comprehensive occupational therapy, patient education, self management, splinting/orthoses, thermotherapy and walking assistive devices. Only “patient education” was recommended in all CPGs. “Adapting the environment” in one.
Conclusion: All six guidelines/recommendations addressed occupational therapy interventions. Two CPGs were referring to the term occupational therapy while the others did not. In all appraised documents no occupational therapist could be identified to be a member of an OA Guideline development group.
Disclosure:
M. Stoffer,
None;
D. Taurok,
None;
B. Prodinger,
None;
J. S. Smolen,
Abbott Immunology Pharmaceuticals, Roche, MSD, Pfizer, UCB, BMS,
2,
Abbott Immunology Pharmaceuticals, Roche, MSD, Pfizer, UCB, BMS, Novo-Nordisk, Lilly, Atsra-Zeneca, Glaxo, Dandoz, Sanofi, Medimmune,
5,
Abbott Immunology Pharmaceuticals, Roche, MSD, Pfizer, UCB, BMS,
,
Rheumatology 5E,
;
A. D. Woolf,
None;
T. A. Stamm,
Abbott Immunology Pharmaceuticals,
5.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/are-occupational-therapy-interventions-included-in-the-most-commonly-used-european-clinical-practice-guidelines-for-the-management-of-osteoarthritis/