Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: There are several instruments to measure disease activity in patients with systemic lupus erythematosus (SLE); however, none of them are able to capture all possible clinical events/manifestations. The LFA-REAL ClinRO has been proposed in order to address this problem as it allows the clinician to evaluate every possible manifestation of SLE. Additionally, the LFA-REAL PRO includes a comprehensive patient-reported disease activity measure. The aim of this study was to determine the correlation between both, the LFA-REAL ClinRO and the LFA-REAL PRO, with other disease activity measures.
Methods: A cross-sectional analyses of patients from a single-center cohort was performed. Disease activity measures included were LFA-REAL ClinRO (0-1400), LFA-REAL PRO (0-1200), SLEDAI-2K, clinical SLEDAI-2K and Physician Global Assessment (PGA, 0-100). The correlation between these indices were evaluated with the Pearson correlation. As an alternative analysis, the correlation between the corresponding domains of LFA-REAL PRO and LFA-REAL ClinRO were examined.
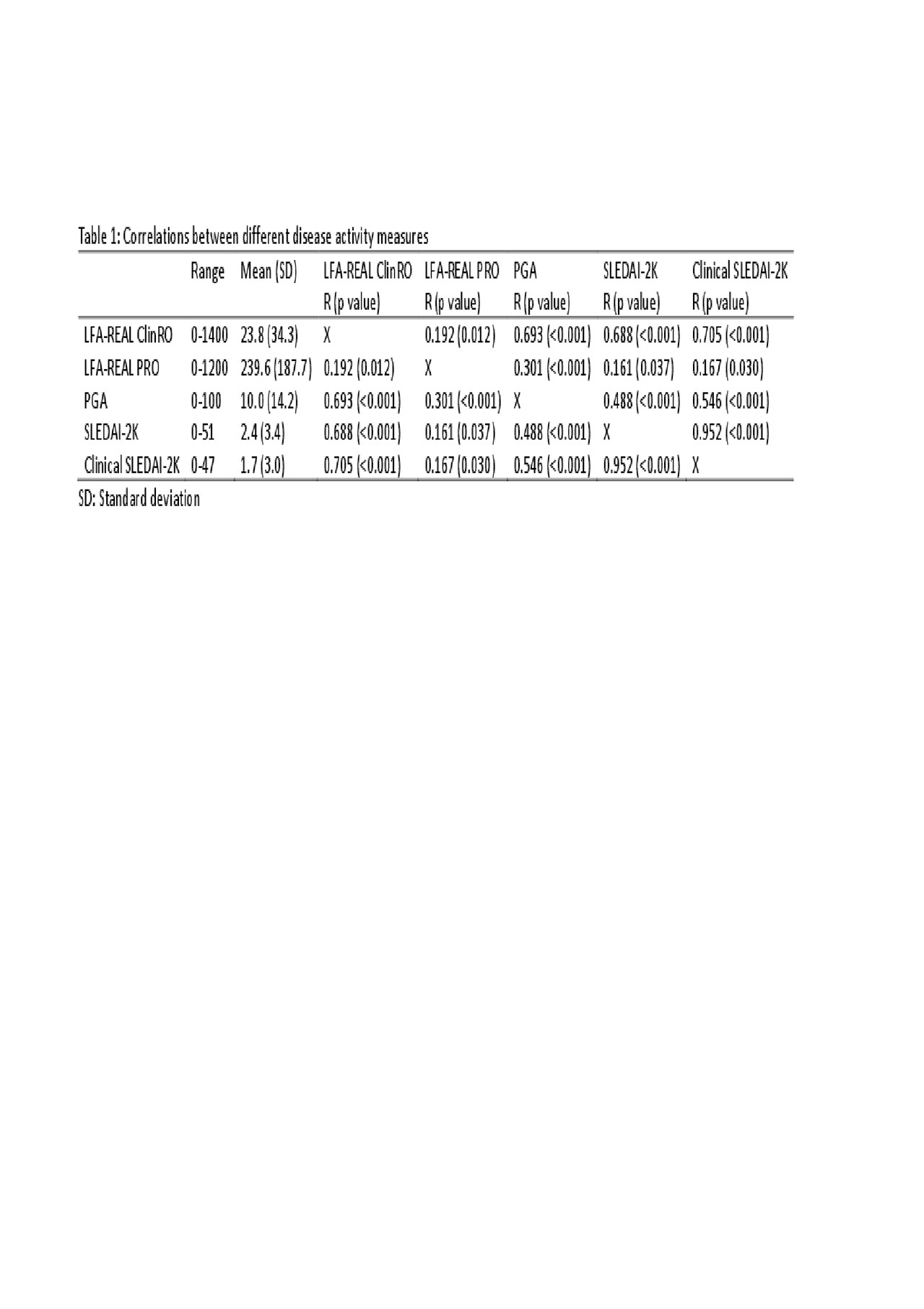
Results: One-hundred and sixty-nine patients with SLE, with a mean age of 45.8 (SD: 14.2), 156 (92.3%) were female. The mean (SD) LFA-REAL ClinRO was 23.8 (34.3), the LFA-REAL PRO was 239.6 (187.7), the PGA was 10.0 (14.2), the SLEDAI-2K was 2.4 (3.4) and the clinical SLEDAI-2K was 1.7 (3.0), the SDI was 1.7 (1.5). LFA-REAL ClinRO correlated with LFA-REAL PRO (R=0.192; p=0.012), PGA (R=0.693, p< 0.001), SLEDAI-2K (R=0.688, p< 0.001) and clinical SLEDAI-2K (R=0.705, p< 0.001); the LFA-REAL PRO correlated with PGA (R=0.301, p< 0.001), SLEDAI-2K (R=0.161, p=0.037) and clinical SLEDAI-2K (R=0.167, p=0.030). The correlations between these disease activity measures are depicted in table 1. Additionally, the LFA-REAL PRO rash correlated with the LFA-REAL ClinRO global mucocutaneous involvement (R=0.154, p=0.046) and with the LFA-REAL ClinRO rash (R=0.245, p=0.001); the LFA-REAL PRO global articular correlated with the LFA-REAL ClinRO global musculoskeletal involvement (R=0.346, p< 0.001).
Conclusion: The LFA-REAL ClinRO and the LFA-REAL PRO had a good correlation with other physician-based disease activity measures. These measures need to be evaluated in other cohorts in order to determine their real value for the monitoring of SLE patients.
To cite this abstract in AMA style:
Ugarte-Gil M, Gamboa-Cárdenas R, Reátegui-Sokolova C, Pimentel-Quiroz V, Zeña-Huancas P, Medina M, Elera-Fitzcarrald C, García S, Gil L, Noriega E, Pastor-Asurza C, Rodríguez-Bellido Z, Merrill J, Askanase A, Alarcón G, Perich-Campos R. Evaluation of the Lupus Foundation of America – Rapid Evaluation of Activity in Lupus (LFA-REAL) Clinician Reported Outcome (ClinRO) and Patient Reported Outcome (PRO) in a Primarily Mestizo Population [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/evaluation-of-the-lupus-foundation-of-america-rapid-evaluation-of-activity-in-lupus-lfa-real-clinician-reported-outcome-clinro-and-patient-reported-outcome-pro-in-a-primarily-mestizo-populatio/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-the-lupus-foundation-of-america-rapid-evaluation-of-activity-in-lupus-lfa-real-clinician-reported-outcome-clinro-and-patient-reported-outcome-pro-in-a-primarily-mestizo-populatio/