Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Inflammatory bowel disease (IBD) is frequently seen in patients with axial spondyloarthritis (axSpA). This study aimed to investigate whether fecal calprotectin levels could predict future development of IBD in axSpA patients.
Methods: This multicenter, prospective observational cohort study used the TReasure database in which web-based registration of rheumatoid arthritis (RA) and SpA patients are being performed in 15 centers across different regions of Turkey. Fecal calprotectin levels were measured in 137 axSpA patients as of September 2018. Fecal calprotectin level ≥200 µg/g was considered significant. All study subjects were evaluated for IBD symptoms (loose defecation, mucous diarrhea, bloody defecation, bloody diarrhea, abdominal pain, obstruction, or pseudo-obstruction). Patients who had previous IBD diagnosis or who used NSAIDs at least 2 weeks before fecal calprotectin measurement were excluded. It was planned to examine patients for IBD by control visits at every 3 months for 1 year. In this paper, preliminary results of the patients were presented.
For axSpA patients, disease activity was evaluated by the ASDAS CRP, BASDAI, BASFI, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), visual analog scale (VAS)-pain, VAS-fatigue, and tender and swollen joint count. Moreover, 24 healthy volunteers and 25 RA patients were included as a control group.
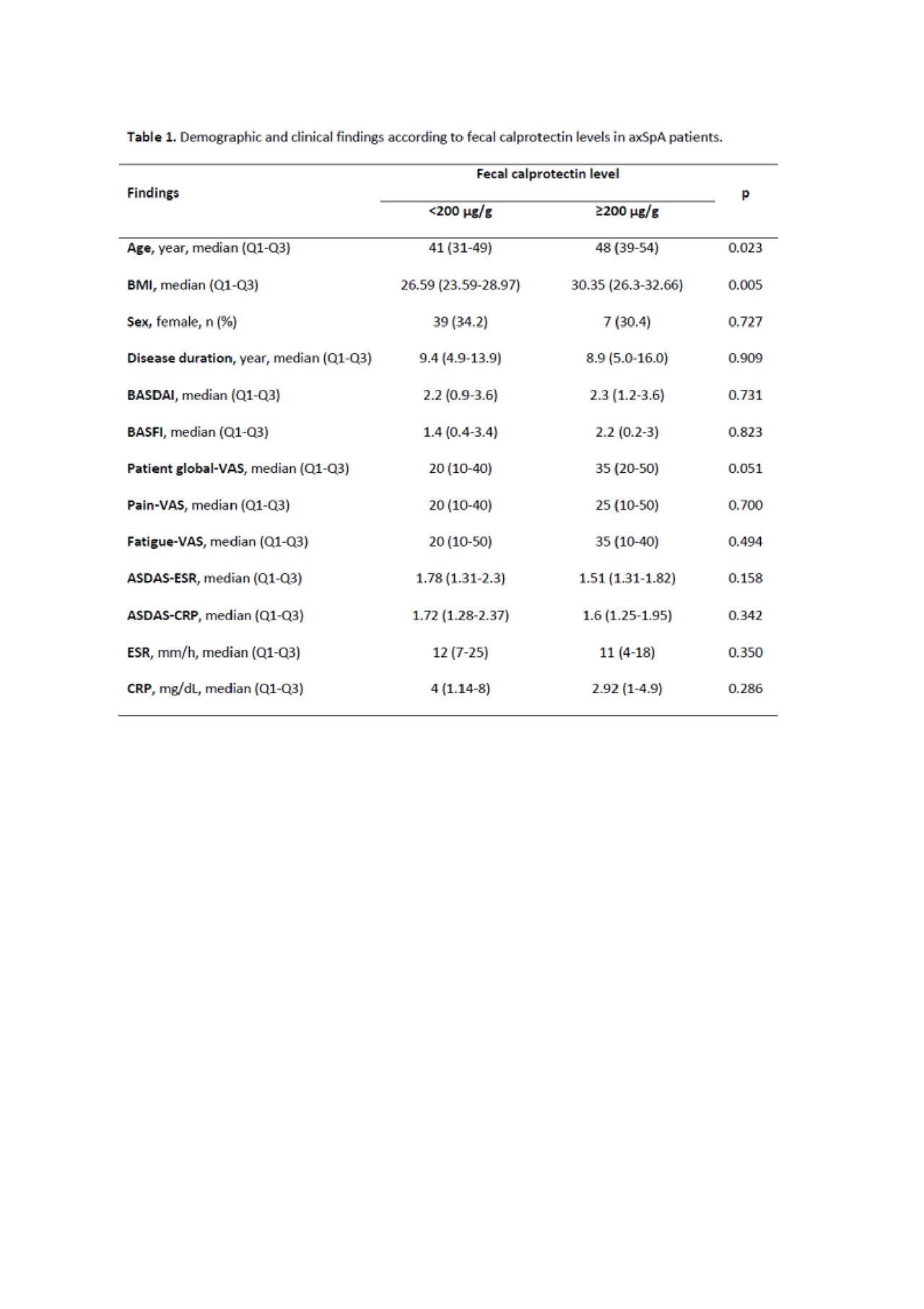
Results: This study included 137 axSpA patients (33.6% females) with the median (Q1-Q3) age of 43 years (33-50 years) and median (Q1-Q3) disease duration of 8.9 years (5.0-13.9 years). Among 24 RA patients (79.2% females), the median (Q1-Q3) age was 59 years (48-64 years) and the median (Q1-Q3) disease duration was 10.6 years (8.1-18.6 years). Rheumatoid factor was positive in 79% of the RA patients. The median (Q1-Q3) fecal calprotectin level was 48 µg/g (30-122 µg/g) in axSpA patients. The median fecal calprotectin level was ≥200 µg/g in 23/137 (16.8%) axSpA patients and in 15/24 (62.5%) RA patients. Fecal calprotectin levels were not high in any healthy volunteers. The axSpA patients with calprotectin level of ≥200 µg/g were older [48 years (39-54 years) vs. 41 years (31-49 years); p=0.025] and had higher BMI values [30.3 (26.3-32.7) vs. 26.6 (23.5-29.0); p=0.003]. Disease activity levels of the patients at baseline are given in Table 1; there was no difference regarding fecal calprotectin level. In the axSpA patients, RA patients, and healthy controls, while the rates of abdominal pain were 5.8% (n=8), 16.5% (n=4), and 8% (n=2), respectively and the rates of loose defecation were 5.1% (n=7), 12.5% (n=3), and 4% (n=1), respectively, none of the patients had mucous diarrhea, bloody diarrhea, and obstruction.
Conclusion: In the present study, preliminary results for the fact that whether or not fecal calprotectin levels could predict future development of IBD were presented. Fecal calprotectin levels were higher in 15% of the asymptomatic axSpA patients; the follow-up results of these patients may give information about daily practice.
To cite this abstract in AMA style:
Yardımcı G, içaçan O, Kabadayi G, Farisoğulları B, Bes C, Akar S, Kalyoncu U. Can Fecal Calprotectin Predict Future Development of Inflammatory Bowel Disease in Axial Spondyloarthritis Patients? – TReasure Real-Life Preliminary Data [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/can-fecal-calprotectin-predict-future-development-of-inflammatory-bowel-disease-in-axial-spondyloarthritis-patients-treasure-real-life-preliminary-data/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/can-fecal-calprotectin-predict-future-development-of-inflammatory-bowel-disease-in-axial-spondyloarthritis-patients-treasure-real-life-preliminary-data/