Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose:
Disease flares in axial spondyloarthritis (axSpA) might occur even in patients with otherwise stable disease receiving effective anti-inflammatory therapy such as TNF inhibitors. The frequency of disease flares, especially in patients with axSpA receiving long-term stable therapy, and factors associated with flares are not sufficiently investigated. The ASAS group recently developed the first data driven definition of a clinically important worsening (flare) in axSpA based on the ASDAS [1]. Objective of the presented analysis was to assess the frequency of disease flares and to identify factors associated with flares in axSpA patients receiving continuous longterm treatment with a TNF Inhibitor.
Methods: In ESTHER, patients with early axSpA (symptom duration ≤5 years) were treated with ETN (n=40) versus sulfasalazine (n=36) for 48 weeks [2]. After one year all patients were treated continuously with etanercept (n=17 patients temporarily interrupted treatment in the 2nd year to assess time to flare and were then (re-)treated with etanercept, except 4 patients who completed the study in sustained remission) for up to 10 years in total. Only patients who were continuously treated with etanercept for at least 6 months were included in the current analysis. The disease flare was defined as a worsening of the ASDAS by ≥0.9 as compared to the value obtained at the previous visit. Univariate and multivariable cox-regression analyses were performed to analyze the predictors of flares.
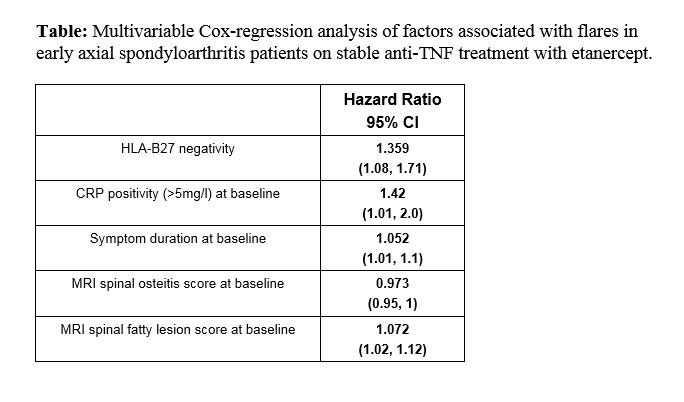
Results: Out of 76 patients who entered the study at baseline, 62 patients (n=32 with radiographic (r-) axSpA and n=30 with non-radiographic (nr-) axSpA) fulfilled the criterion of the continuous etanercept treatment. A total of 22 patients (35%) experienced at least one flare over the entire treatment period 10 patients (31.3%) in the r-axSpA and 12 patients (40%) in the nr-axSpA subgroup) – figure. A total of 81 flares occurred (33 and 48 in the r- and nr-axSpA subgroups, respectively) in the 10 years of follow-up. None of the documented disease flares resulted in a direct study withdrawal. The majority of flares occurred within first 4 years of treatment (figure). There were also no statistically significant differences between nr- and r-axSpA in the time until the first flare (p=0.4, Log-rank test). In the multivariable Cox regression analysis, an elevated CRP value ( >5mg/l) at baseline, HLA-B27 negativity, a longer symptom duration at study entry, a lower spinal osteitis score and a higher spinal fatty lesion score on MRI at baseline were associated with a higher risk for flares (Table).
Conclusion: Disease flares according to the ASAS definition of clinically important worsening in axSpA based on ASDAS occurred in approximately one third of patients with early axSpA who received a treatment with the TNF-inhibitor etanercept for up to 10 years without major differences between r- and nr- forms of axSpA. HLA-B27 negativity, elevated CRP at baseline, longer symptom duration with higher fatty lesion and lower osteitis spinal score were associated with a higher risk of flares.
MT work at Charité was supported by an award from TUBITAK.
1. Molto et al. Ann Rheum Dis. 2018 Jan; 77(1):124-127.
2. Song IH, et al. Ann Rheum Dis. 2011 Apr; 70(4):590-596.
To cite this abstract in AMA style:
Proft F, Torgutalp M, Weiß A, Protopopov M, Rios Rodriguez V, Haibel H, Hermann K, Althoff C, Behmer O, Sieper J, Poddubnyy D. Frequency of Disease Flares Under Long-Term Anti-TNF Therapy in Patients with Early Axial Spondyloarthritis: Results from the Etanercept versus Sulfasalazine in Early Axial Spondyloarthritis Trial (ESTHER) [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/frequency-of-disease-flares-under-long-term-anti-tnf-therapy-in-patients-with-early-axial-spondyloarthritis-results-from-the-etanercept-versus-sulfasalazine-in-early-axial-spondyloarthritis-trial-es/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/frequency-of-disease-flares-under-long-term-anti-tnf-therapy-in-patients-with-early-axial-spondyloarthritis-results-from-the-etanercept-versus-sulfasalazine-in-early-axial-spondyloarthritis-trial-es/