Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Early treatment of RA within the therapeutic window (0-3 months from symptom onset), has been associated with improved clinical outcomes and physical function. However, ≤42% of RA patients (pts) visit a rheumatologist within 90 days of symptom onset1,2. Upadacitinib (UPA), an oral, reversible, potent JAK-1 selective inhibitor3 demonstrated efficacy in pts with moderate to severely active RA who were MTX‑naïve or had an inadequate response to csDMARDs/bDMARDs4-6.
Methods: In SELECT–EARLY, MTX-naïve pts with active RA and poor prognosis were randomized 1:1:1 to once‑daily UPA monotherapy at 15 or 30 mg or weekly MTX (titrated up to 20 mg/week through Week 8). Efficacy (including ACR, DAS28(CRP), CDAI responses and change in mTSS) and safety outcomes from a post-hoc analysis of patients who received treatment within 90 days from diagnosis are reported here. The statistical significance defined as p< 0.05 was exploratory in nature.
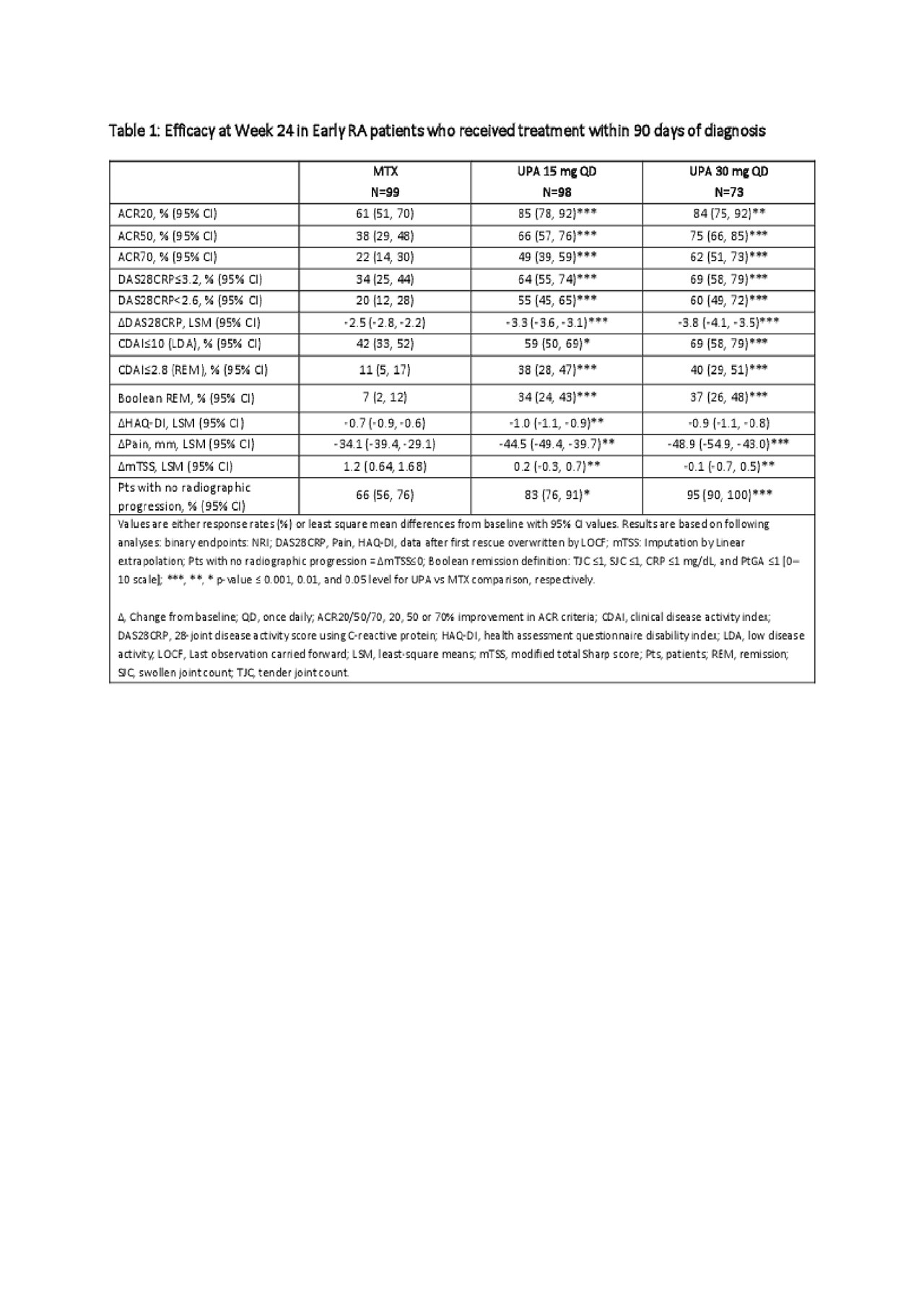
Results: A total of 270 pts commenced treatment within 90 days from RA diagnosis (median: 44 days [11, 89]). Pts in each arm were mostly female (70%), had moderately to severely active RA with mean DAS28(CRP) =5.9±1.02, had structural joint damage (mean mTSS =7.7±21.5) and were seropositive for both ACPA and RF at baseline (72%)4. At Week 24, compared to MTX, significantly greater proportions of pts receiving UPA 15 or 30 mg monotherapy achieved efficacy outcomes including ACR20, 50 and 70 responses, DAS28CRP< 2.6, CDAI≤2.8 or Boolean remission. Improvements in physical function (HAQ-DI) and decrease in pain were also significantly greater in pts receiving UPA 15 and 30 mg vs MTX at Week 24. Treatment with UPA was also associated with a greater inhibition of structural joint damage compared with MTX (Table 1). Safety outcomes were consistent with the full study and the integrated safety analysis (all phase 3 studies of UPA). Compared to MTX, higher frequencies of serious infections and herpes zoster were reported in both UPA groups. There were 2 deaths in total (UPA 30 mg: 1 due to cardiovascular death and 1 due to pneumonia and sepsis) (Table 2).
Conclusion: In RA pts, early initiation of treatment with UPA 15 mg and 30 mg monotherapy within 3 months from diagnosis was associated with clinically meaningful improvements in efficacy, including remission and inhibition of progression of structural joint damage compared to MTX. The safety profile was consistent with the overall study and the integrated phase 3 safety analysis7. UPA was more effective than MTX in enabling more patients to reach their treatment targets of remission or low disease activity when treated within 3 months of diagnosis.
References:
- Raza K et al. Ann Rheum Dis. 2011;70(10):1822-5.
- Stack RJ et al. BMJ Open. 2019;9:
- Parmentier et al. BMC Rheumatol. 2018;2:23.
- van Vollenhoven R et al, Arth Rheumatol. 2018; 70 (s10) [Abs ACR2018].
- Burmester GR et al. Lancet 2018;391:2503-12.
- Genovese MC et al, Lancet 2018;391:2513-24.
- Cohen S et al, Ann Rheum Dis [Abs EULAR2019].
To cite this abstract in AMA style:
Kapetanovic M, Andersson M, Friedman A, Shaw T, Song Y, Aletaha D, Buch M, Müller-Ladner U, Pope J. Efficacy and Safety of Upadacitinib Monotherapy in MTX-naïve Patients with Early Active RA Receiving Treatment Within 3 Months of Diagnosis: A Post-hoc Analysis of the SELECT-EARLY [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-upadacitinib-monotherapy-in-mtx-naive-patients-with-early-active-ra-receiving-treatment-within-3-months-of-diagnosis-a-post-hoc-analysis-of-the-select-early/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-upadacitinib-monotherapy-in-mtx-naive-patients-with-early-active-ra-receiving-treatment-within-3-months-of-diagnosis-a-post-hoc-analysis-of-the-select-early/