Session Information
Date: Sunday, November 10, 2019
Title: Patient Outcomes, Preferences, & Attitudes Poster I: Patient Reported Outcomes
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Physical function and health-related quality of life (HRQoL) are negatively impacted in patients (pts) with PsA, and treatment with conventional and biological (b) DMARDs improved patient-reported outcomes (PROs), including HRQoL measures. The objective of this analysis was to assess the impact of adalimumab (ADA) vs placebo (PBO) on PROs following 12-week (wk) treatment in the randomized-controlled ADEPT trial.
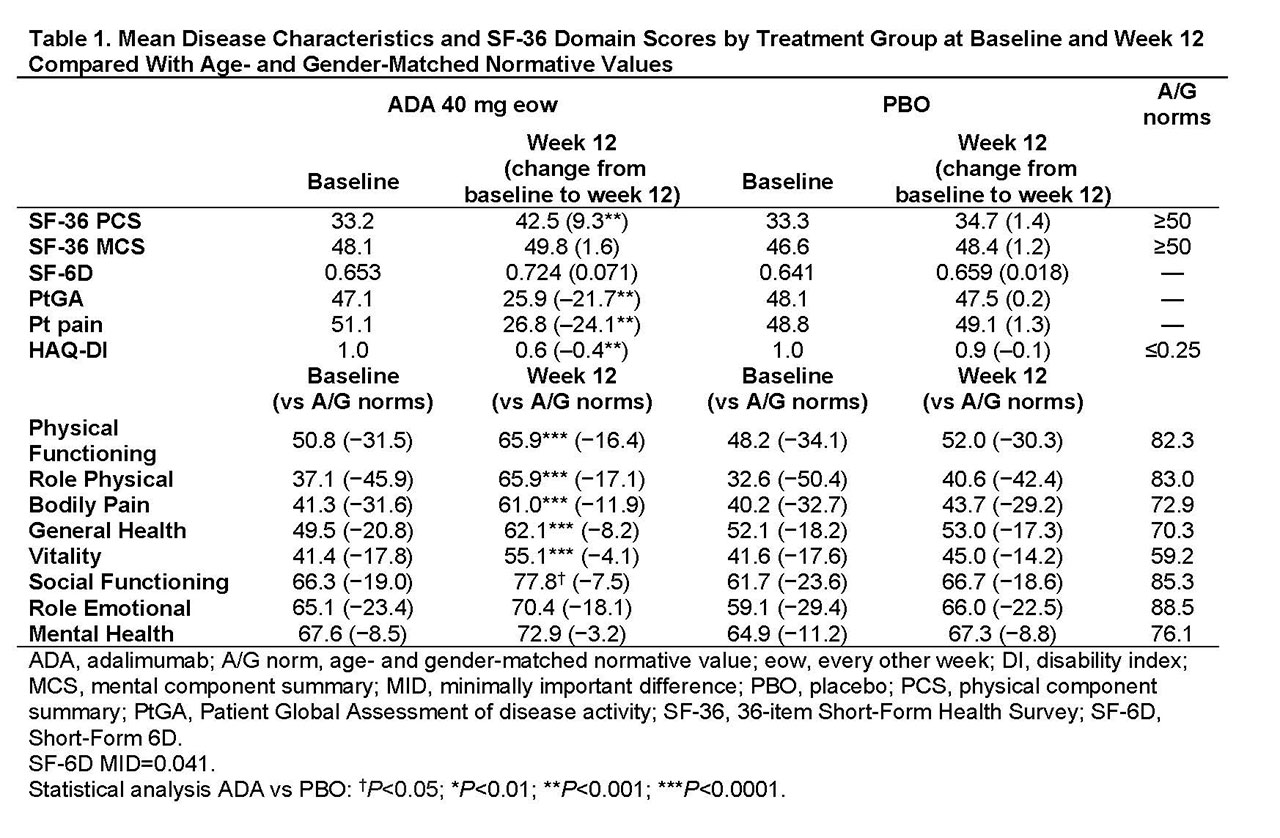
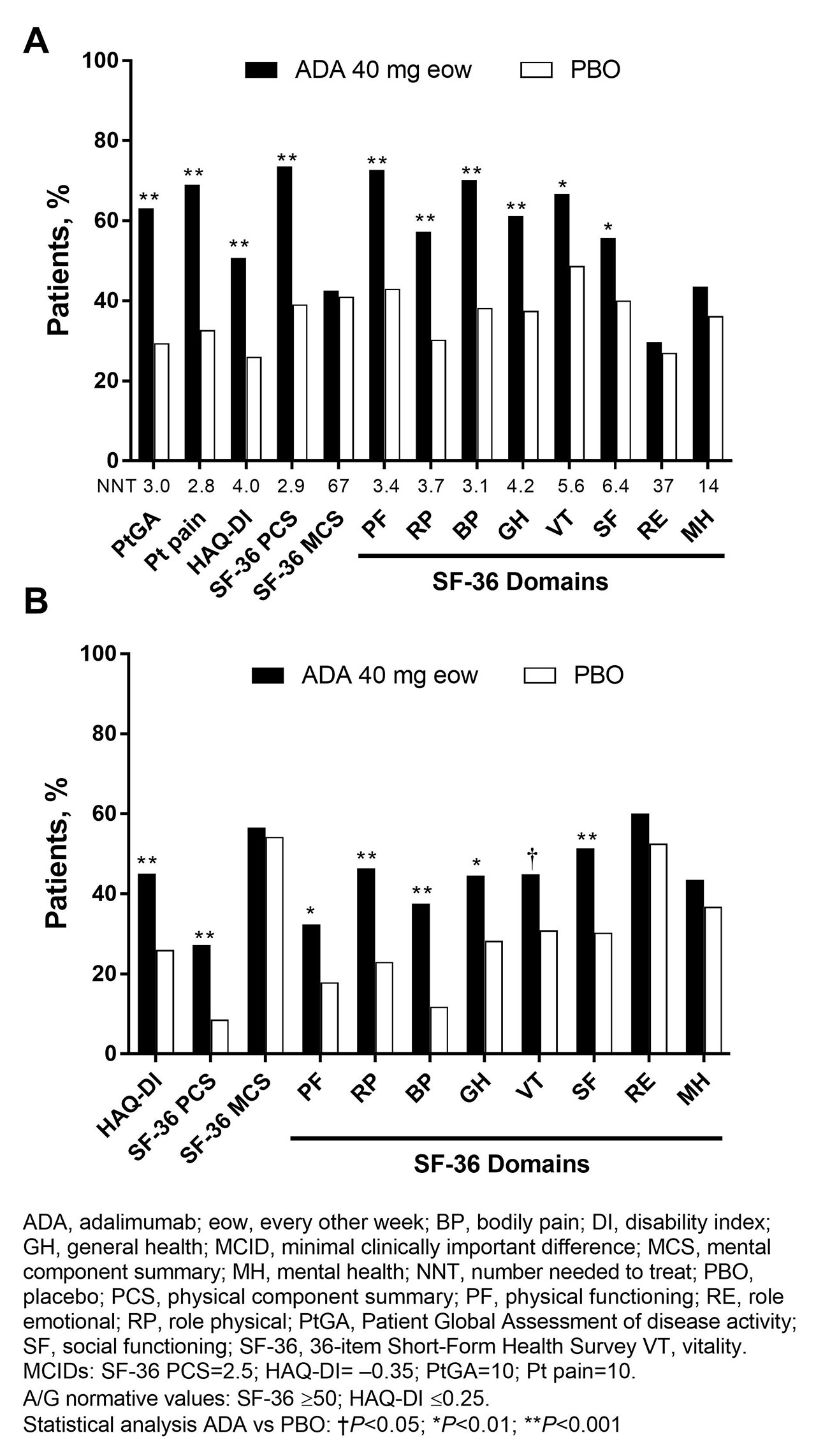
Methods: Pts (n=315) with moderately to severely active PsA who were bDMARD naive (50% were receiving MTX) were randomized to ADA 40 mg or PBO every other wk. This analysis assessed PROs at baseline (BL) and wk 12 using the 36-item Short-Form (SF-36) Health Survey physical (PCS) and mental component summary (MCS) scores, 8 domain scores ranging from 0 (worst) to 100 (best), and the SF-6D utility measure derived from all 8 SF-36 domains with scores ranging from 0.296 (worst) to 1.00 (full health) and a minimally important difference (MID) of 0.041. Patient Global Assessment of disease activity (PtGA) and pain (both utilizing 100 mm visual analog scale [VAS]) and HAQ disability index (DI) also were assessed. Mean changes from BL, percentages of pts with improvements ≥minimum clinically important differences (MCID), and scores ≥US age- and gender-matched normative values (A/G norms) were analyzed, based on as observed data. P values were assessed by analysis of variance model for continuous variables and Cochran–Mantel–Haenszel test for binary outcomes, adjusting by baseline MTX use and extent of psoriasis; no P value adjustments for multiplicity were performed. Numbers needed to treat (NNTs) are reported using proportions of pts reporting improvements ≥MCID in SF-36, PtGA, pain, and HAQ-DI.
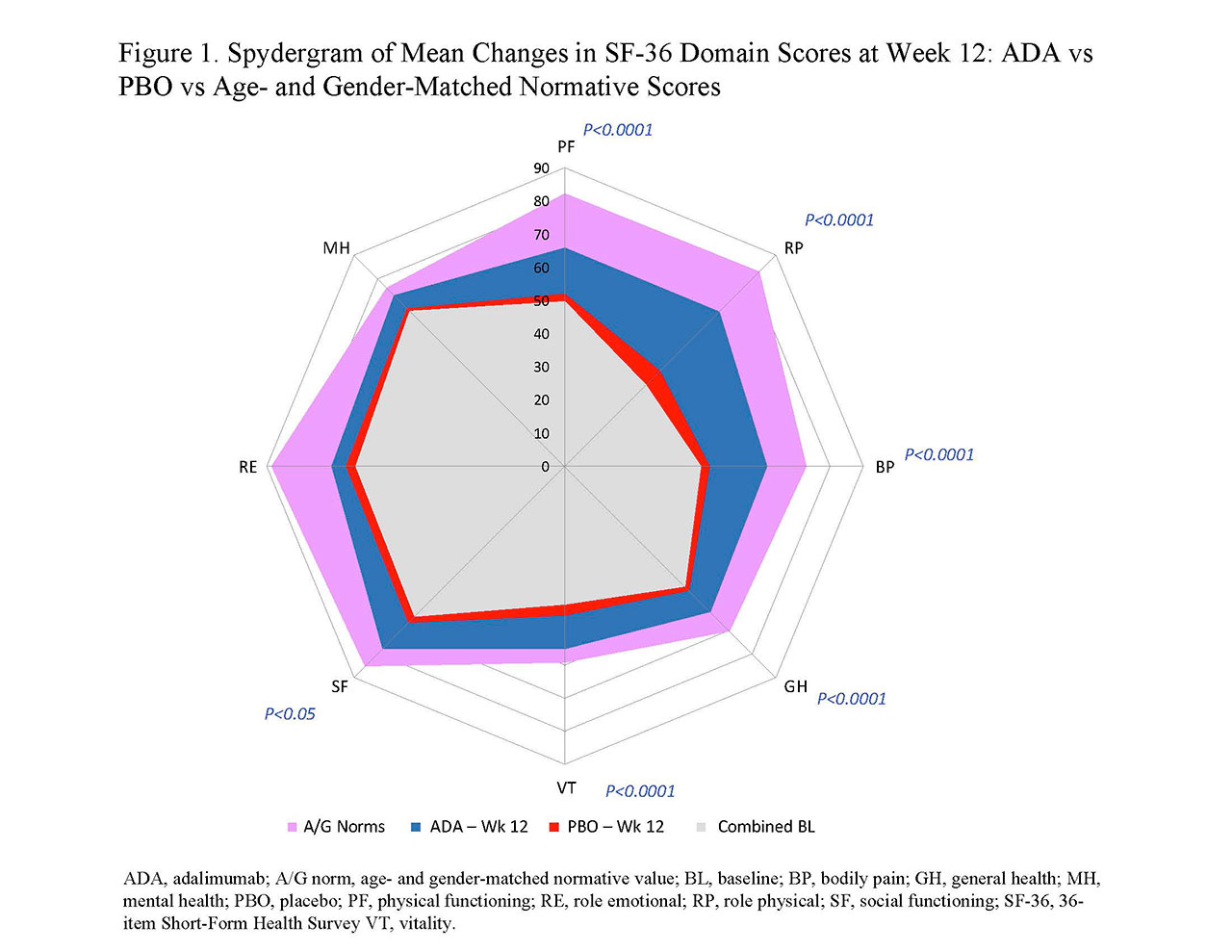
Results: In general, BL PRO scores were similar between ADA (n=151) and PBO (n=162; Table 1). Improvements from BL at wk 12 with ADA vs PBO were significant in PtGA, pain, HAQ-DI, and SF-36 PCS (change: 9.3 vs 1.4; P< 0.001) but not in SF-36 MCS (1.6 vs 1.2; Table 1). Six of 8 SF-36 domains (physical functioning, role physical, bodily pain, general health, social functioning, and vitality) significantly improved from BL to wk 12 with ADA vs PBO (all P≤0.05; Table 1 and Figure 1). SF-6D improvements exceeded MID with ADA (change: 0.071) vs PBO (0.018); the ADA SF-6D score (0.724) also approached the A/G norm of 0.778 at wk 12 (Table 1). Proportions of pts reporting improvements ≥MCID at wk 12 were significantly greater with ADA vs PBO in all PROs, except SF-36 role emotional and mental health domains, with corresponding NNTs ≤6.4 (Figure 2). Proportions of pts who reported scores ≥A/G norms in HAQ-DI, SF-36 PCS, and 6 of 8 SF-36 domains were significantly greater with ADA vs PBO (Figure 2).
Conclusion: Statistically significant and clinically meaningful improvements and scores ≥A/G norms (higher definition of response) at week 12 were reported with ADA treatment vs PBO in pts with moderately to severely active PsA. These data are consistent with improvements in PROs reported with other bDMARDs in patients with PsA.
Acknowledgment: Medical writing support was provided by M Hovenden and J Matsuura of Complete Publication Solutions, LLC (North Wales, PA) and was funded by AbbVie.
To cite this abstract in AMA style:
Strand V, Patel P, Chen N, Lesser E. The Impact of Adalimumab vs Placebo on Patient-Reported Outcomes and Utility Measures Among Patients with Moderately to Severely Active Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/the-impact-of-adalimumab-vs-placebo-on-patient-reported-outcomes-and-utility-measures-among-patients-with-moderately-to-severely-active-psoriatic-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-impact-of-adalimumab-vs-placebo-on-patient-reported-outcomes-and-utility-measures-among-patients-with-moderately-to-severely-active-psoriatic-arthritis/