Session Information
Date: Sunday, November 10, 2019
Title: Measures Of Healthcare Quality Poster I: Testing, Screening, & Treating
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
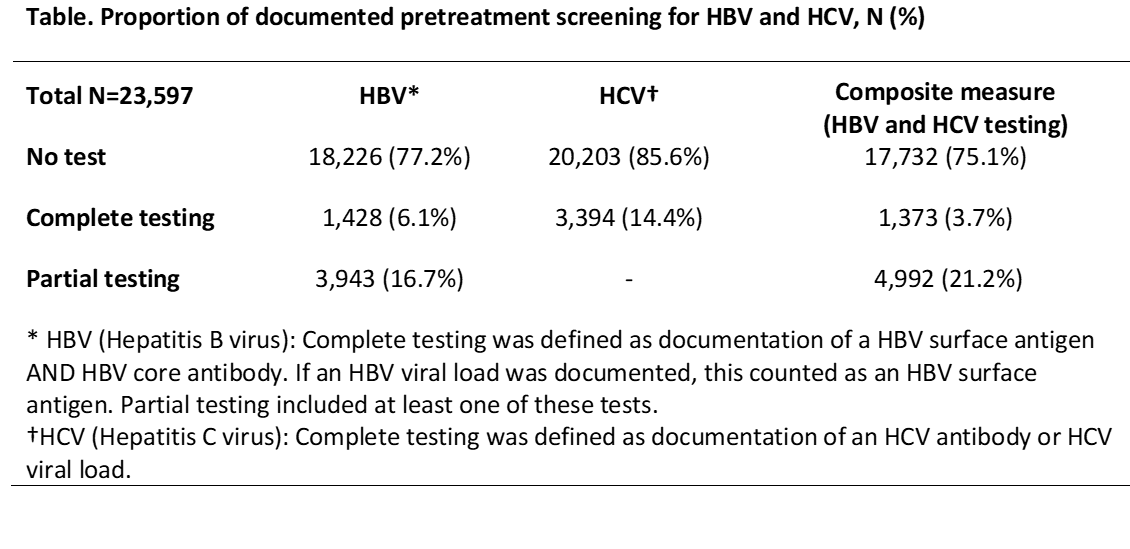
Background/Purpose: Testing for hepatitis B virus (HBV) and hepatitis C virus (HCV) is recommended for patients initiating biologics or new synthetic DMARDs, which can increase the risk of viral hepatitis reactivation. We examined pre-treatment screening for HBV and HCV among practices participating in ACR’s RISE registry for patients who were new users of biologics or new synthetic DMARDs.
Methods: Data derived from Rheumatology Informatics System for Effectiveness (RISE), a national EHR-enabled rheumatology registry that passively collects data on all patients seen by participating practices. As of 2017, RISE held validated data from 1,257 providers in 236 practices, representing an estimated 36% of the U.S. clinical rheumatology workforce. Patients included in this study were ≥ 18 years old and had ≥ 1 prescription for a biologic or new synthetic DMARD between Jan 1 and Dec 31, 2017; the “index date” was defined as the first prescription date. New medication users were identified if they had ≥ 2 visits in the 12 months prior to their index date without any biologic or new small synthetic DMARD use. HBV screening was defined as “complete” if HBV surface antigen AND HBV core antibody were documented or “partial” if only one test was documented; HBV viral load satisfied the HBV surface antigen requirement. HCV screening was defined as having a documented HCV antibody or viral load test. We assessed screening across several windows (before the index date; allowing for a 60-day grace period after the index date; or allowing up to a 1-year grace period after the index date). We assessed practice-level performance for practices reporting on ≥ 20 patients.
Results: 23,597 patients were included from 196 practices. 71.9% were female, mean age of 57.3±14.4 years. 37% were non-white, including 6.9% Hispanic. The most common class of medication was TNFi (62.9%). Overall, only 22.8% patients had any documented HBV screening and 14.4% had any documented HCV screening (Table). Among those with complete testing, most screening was completed prior to the index date (82.6%); 96.9% completed screening by 60 days after the index date. Patterns were similar for HCV screening. Among 168 practices in the practice-level analysis, median performance was 0% (range 0-62.7%) for HBV, 0% (range 0-80.4%) for HCV screening, and 0% (range 0-62.7%) for the composite (HBV and HCV) screening measure.
Conclusion: Only a small proportion of RISE patients who were new users of biologics or new synthetic DMARDs had documented screening for HBV or HCV. It is likely that some patients were tested outside the rheumatology practice and also possible that results are documented in clinical notes or scanned documents, which are not currently accessible for use in electronic clinical quality measures. In order to meet criteria for measures that assess performance of pre-treatment HBV and HCV screening, practices will need to adjust workflows to ensure these patient safety measures are captured consistently in structured fields within the electronic health record.
Disclaimer: This data was supported by the ACR’s RISE Registry. However, the views expressed represent those of the authors, not necessarily those of the ACR.
To cite this abstract in AMA style:
Li J, Kay J, Yazdany J, Schmajuk G. Pre-treatment Screening for Hepatitis B and C Among Users of Biologics or New Synthetic Disease Modifying Drugs: An Analysis Using RISE Data [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/pre-treatment-screening-for-hepatitis-b-and-c-among-users-of-biologics-or-new-synthetic-disease-modifying-drugs-an-analysis-using-rise-data/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pre-treatment-screening-for-hepatitis-b-and-c-among-users-of-biologics-or-new-synthetic-disease-modifying-drugs-an-analysis-using-rise-data/