Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: While Medicare’s Part D prescription drug benefit expanded access to drugs for many patients, it also includes a controversial provision called the “coverage gap” during which beneficiaries are fully responsible for drug costs. As an initial step in understanding the potential impact of cost-sharing for beneficiaries with rheumatoid arthritis (RA), we compared drug utilization and out-of-pocket (OOP) costs for biologic and non-biologic disease-modifying anti-rheumatic drugs (DMARDs) for two groups of patients: those with a coverage gap and those who were not subject to cost-sharing because they were fully eligible for the low-income subsidy program.
Methods : Data derive from Medicare fee-for-service claims files for 2009 for a 5% random sample of beneficiaries linked to the Medicare Part D prescription drug event file. We included 5,808 individuals age ≥ 65 years with ≥ 2 face-to-face encounters for RA who were continuously enrolled in a Part D drug plan and had at least one prescription DMARD event. We compared patients who had no, partial or full coverage during the gap phase to those who were fully eligible for the low-income subsidy program, which is intended to reduce OOP costs for those with low socioeconomic status. We calculated mean annual RA drug OOP costs per patient for the deductible, initial, gap, and catastrophic phases of Part D coverage in two mutually exclusive groups: 1) those using biologic DMARDs (with or without non-biologic DMARDs) and 2) those using only non-biologic DMARDs.
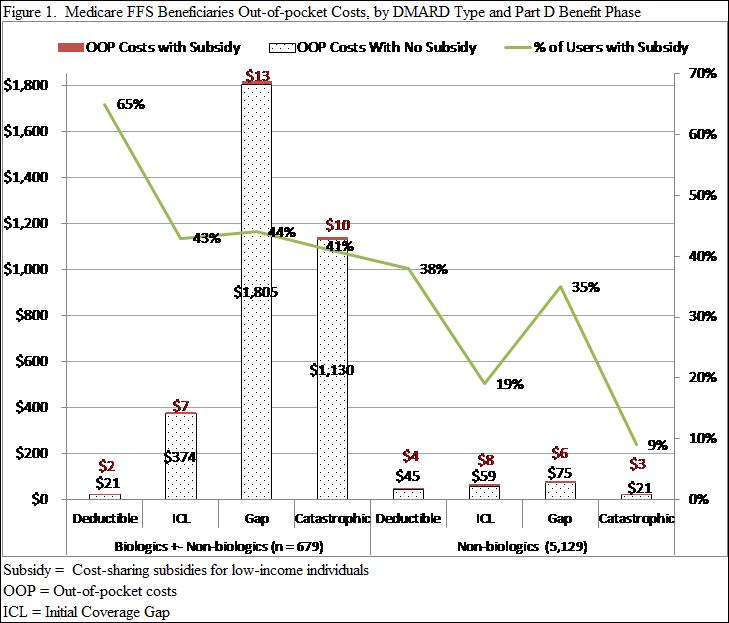
Results : Among the 5,808 RA beneficiaries with Part D drug events for DMARDs in 2009, 679 (12%) were classified as biologic DMARD users, 5,129 (88%) as non-biologic DMARD users and 1,414 (24%) received a low-income subsidy. 44% of biologic DMARD users received a low-income subsidy compared to 22% of non-biologic users. For the low-income subsidy group, OOP annual costs for biologic and non-biologics were low as expected ($26, $11, respectively) and varied little over the benefit phases (range $2-13; see Figure). The non-subsidized group’s annual OOP costs for biologics and non-biologics were $3,009 and $85, respectively; larger variations in OOP cost existed for biologics ($21 to $1,805) compared to non-biologics ($21 to $75) across the benefit phases (Figure) with the highest costs incurred during the gap phase.
Conclusion : We found that a significantly larger proportion of biologic DMARD users were enrolled in the low-income subsidy program compared to non-biologic DMARD users. Whether the low rates of biologic DMARD use among Medicare beneficiaries who are not eligible for financial subsidies is a consequence of substantial cost-sharing warrants further investigation.
Disclosure:
C. Tonner,
None;
G. Schmajuk,
None;
J. Yazdany,
None.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cost-sharing-and-utilization-of-biologic-and-non-biologic-dmards-among-u-s-medicare-beneficiaries-with-rheumatoid-arthritis/