Session Information
Date: Wednesday, October 24, 2018
Title: 6W007 ACR Abstract: Patient Outcomes, Preferences, & Attitudes II: PROs (2910–2915)
Session Type: ACR Concurrent Abstract Session
Session Time: 9:00AM-10:30AM
Background/Purpose: Randomized controlled trials currently report benefits and adverse events (AEs) separately, and therefore do not permit comparisons of patients’ overall experiences on one treatment versus another. The purpose of this study is to develop a Global Patient-Reported Outcome Measure (G-PROM) to quantify and compare the distribution of patients’ overall experiences on medications.
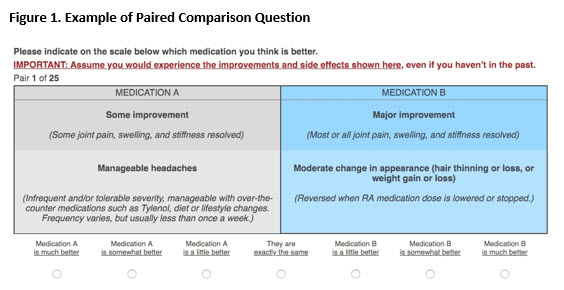
Methods: We invited rheumatoid arthritis (RA) patients who were part of an online community to complete a survey, based on Trajectory Mapping (TM) to generate a hierarchy of AEs. The TM survey establishes a hierarchy by enabling patients to indicate whether an AE is worse, better, or no better or worse than a referent AE. TM allows the construction of “equivalence classes” i.e., groups of AEs judged by patients as having a comparable impact on quality of life. We subsequently conducted a second survey in which participants (who did not participate in the initial TM survey) were asked to indicate their preference for pairs of outcomes, where each outcome include both a specified level of benefit [little or no improvement (ACR20 or less), some improvement (between ACR20 and 50), and major improvement (ACR50 and greater)] and an AE (see Figure 1).
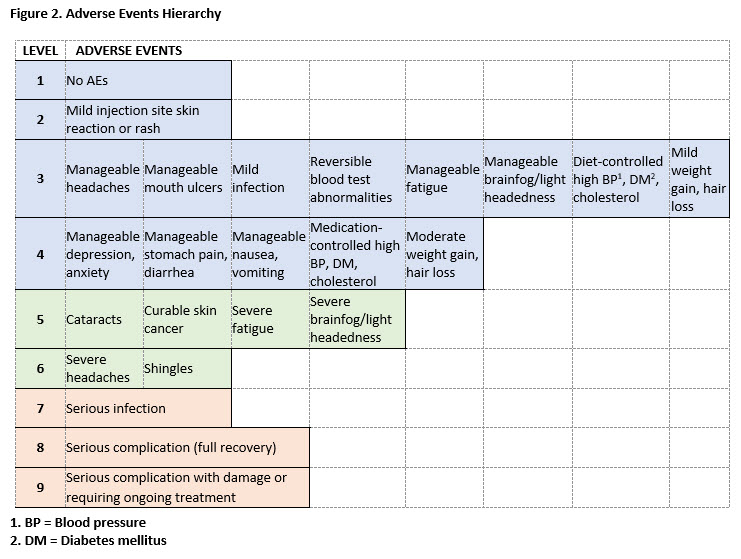
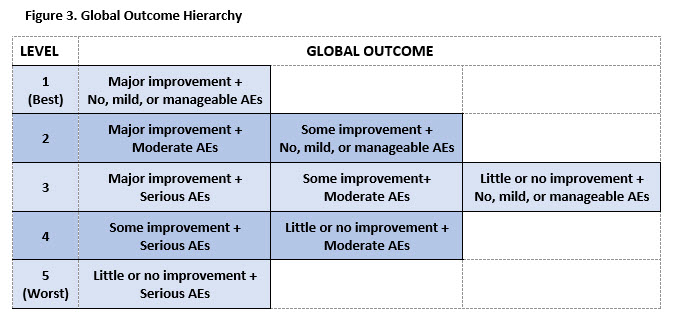
Results: 195 participants completed the initial TM survey. The mean age was 53.5 (11.6), 89% were female, and 56% were college graduates. The initial TM survey generated 11 hierarchies of AEs. The final hierarchy (9 levels of AEs ranging from no AEs to serious AEs resulting in irreversible harm) was chosen based on goodness of fit parameters (see Figure 2). 426 participants with similar demographic characteristics completed the second survey. Ratings revealed that when paired with benefits, AEs clustered into 3 main groups: no, mild or manageable AEs (Levels 1-4), moderate AEs (Levels 5-6), and serious AEs (Levels 7-9). Participants’ ratings generated a 5-level hierarchy of global outcomes illustrated in Figure 3.
Conclusion: After validation, G-PROM will enable randomized controlled trials to report the percentage of patients classified into each level; thus, providing patients and their rheumatologists with a much clearer understanding of the range and likelihood of the total effects of competing treatment options on their quality of life.
To cite this abstract in AMA style:
Fraenkel L, Nowell WB, Wiedmeyer C, Wei Z, Michaud K, Neogi T, Ramsey C, Broniatowski D. Development of a Rheumatoid Arthritis Global Outcome Measure to Enable Comparisons of Patient Experiences across Treatment Arms in Randomized Clinical Trials [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/development-of-a-rheumatoid-arthritis-global-outcome-measure-to-enable-comparisons-of-patient-experiences-across-treatment-arms-in-randomized-clinical-trials/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-a-rheumatoid-arthritis-global-outcome-measure-to-enable-comparisons-of-patient-experiences-across-treatment-arms-in-randomized-clinical-trials/