Session Information
Date: Tuesday, October 23, 2018
Title: 5T109 ACR Abstract: RA–Treatments V: Beyond Individual Compounds (2874–2879)
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose:
Phase 3 (P3) clinical trials are the mainstay of drug development in all areas of medicine, including rheumatology, allowing to determine safety and efficacy of new drugs on their way to approval. Historically, efficacy results of P3 trials have often been disappointing with respect to the expectations set by phase 2 (P2) trials. It is unclear whether these observations are reflection of a true bias or merely a play of chance. We therefore systematically compared efficacy of P2 versus P3 trials in RA.
Methods:
We performed a systematic review of all disease modifying anti-rheumatic drugs (DMARDs) tested in P2 trials in rheumatoid arthritis (RA) over the last 20 years for which also P3 trials exist. We searched Medline, EMBASE, and the Cochrane Library to identify all randomized controlled double-blind trials investigating biological (b) and targeted synthetic DMARDs in RA. The criteria for inclusion in the analyses were defined as follows: (i) treatment arms in P2 and P3 used the same treatment regimen; (ii) the same RA population was studied (DMARD naïve; conventional DMARD insufficient responders (IR); bDMARD IR). The treatment regimen was regarded the same when a DMARD was used at the same dose, interval and route in P2 and P3. Multilevel mixed model logistic regression was used to determine a summary estimate for comparison of P2 versus P3 results on the outcomes of American College of Rheumatology (ACR)20, ACR50, and ACR70 responses (in separate models). Results are expressed as Odds Ratio (OR) and 95% confidence intervals (95% CI).
Results:
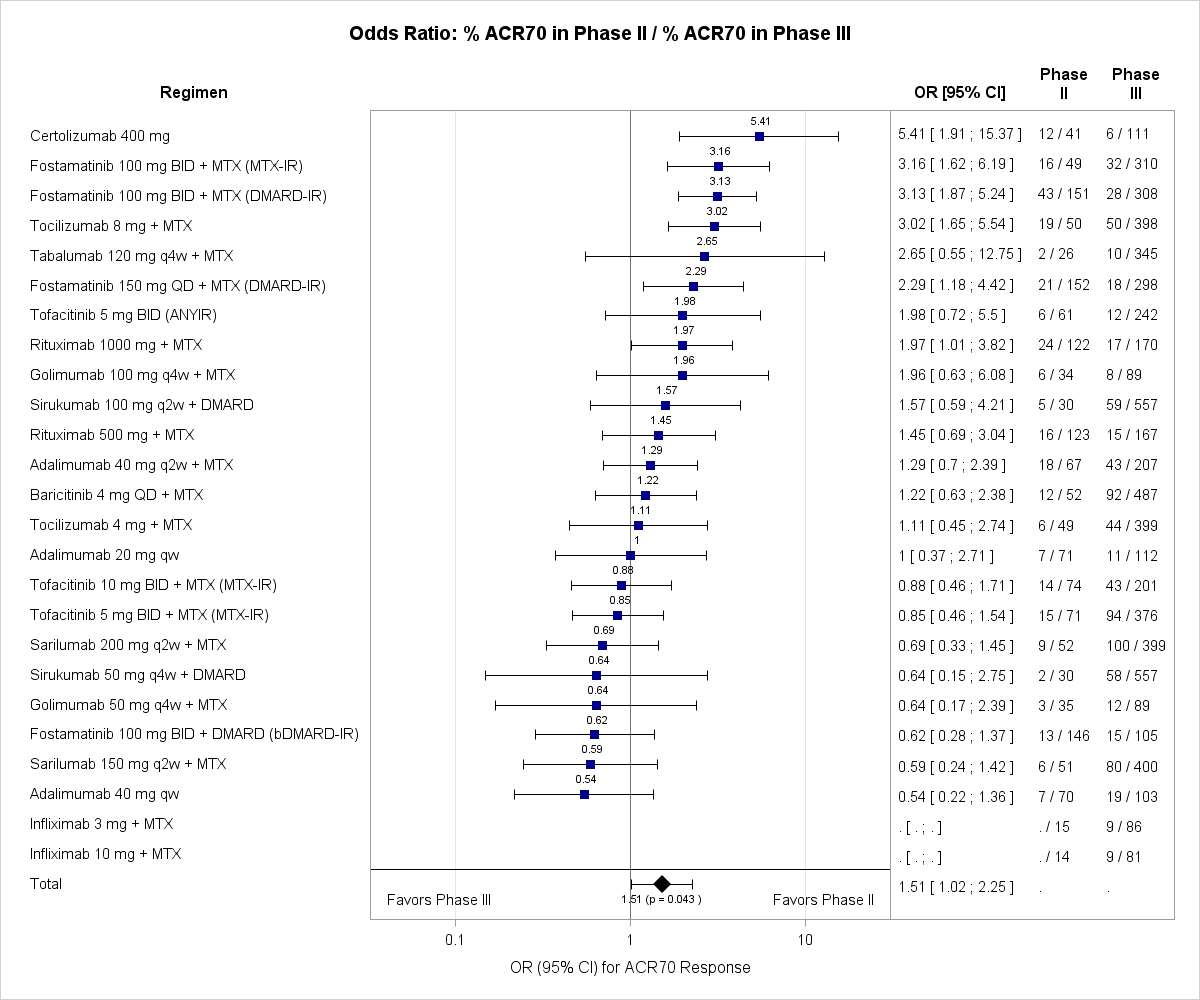
In total 1290 abstracts were screened of which 133 were regarded as potentially relevant, with 34 trials (16 agents, 25 regimens, 8855 patients) finally included in the analysis. Summary estimates revealed that outcomes of P2 trials were systematically overestimating the subsequent P3 results for ACR20 (OR: 1.45; 95% CI: 1.12-1.88; p=0.010), ACR50 (OR: 1.45; 95% CI: 1.09-1.93; p=0.017) and ACR70 (OR: 1.51; 95% CI: 1.02-2.25; p=0.043). Figure 1 shows ORs (95% CI) for ACR70 responses, which represent the most relevant clinical outcome in RA.
Conclusion:
Our results reveal that Phase 2 clinical trials overestimate the treatment effects when compared with subsequent Phase 3 trials in RA. The identification of this systematic bias towards overestimation of efficacy by Phase 2 studies has implications for considerations of clinical investigators, sponsors, and regulatory agencies during the development and licencing process of new compounds, as well as potential ethical implications. Investigation of determinants of Phase 2 overestimation is currently ongoing.
To cite this abstract in AMA style:
Kerschbaumer A, Herkner H, Smolen JS, Aletaha D. Phase II Clinical Trials Systematically Overestimate Treatment Effects of Subsequent Phase III Trials in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/phase-ii-clinical-trials-systematically-overestimate-treatment-effects-of-subsequent-phase-iii-trials-in-rheumatoid-arthritis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/phase-ii-clinical-trials-systematically-overestimate-treatment-effects-of-subsequent-phase-iii-trials-in-rheumatoid-arthritis/