Session Information
Date: Tuesday, October 23, 2018
Title: 5T106 ACR Abstract: Measures of Healthcare Quality I: QI in RA (2856–2861)
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: The American College of Rheumatology has published guidelines for laboratory monitoring of conventional DMARDs (cDMARDs). At Kaiser Permanente Colorado (KPCO), although most patients eventually complete recommended monitoring, we found a test utilization gap in timeliness to monitoring. The purpose of this work was to develop a laboratory-led intervention to improve timeliness of test completion. Here we describe the direct-to-patient interactive voice response (IVR) outreach we developed and present healthcare system results of this work.
Methods: KPCO is an integrated healthcare delivery system with about 600,000 members in the Denver-Boulder area. The KPCO Virtual Data Warehouse (VDW) was used to identify patients and to extract all data. cDMARDs included were: methotrexate (MTX), leflunomide (LEF), sulfasalazine (SZA), and azathioprine (AZA). Biologic DMARDs (bDMARDs) included were: tocilizumab (TCZ), tofacitinib (TOF), adalimumab (ADA), certolizumab pegol (CER), etanercept (ETN), golimumab (GOL), infliximab (IFX) and rituximab (RTX). We identified members aged ≥18 receiving cDMARD/bDMARD therapy from a rheumatologist. Patients were eligible for outreach if they were due (within 3 days) or overdue for testing based on their most recent dates of alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (Cr), and/or complete blood count (CBC) testing. Whether patients were due/overdue was defined as: 1) MTX, LEF, SZA, AZA: ALT, AST, Cr and CBC every 4 weeks if <3 months therapy; every 12 weeks if ≥3 months therapy. 2) TCZ, TOF: ALT, AST, Cr, and CBC every 6 weeks if <3 months therapy; every 12 weeks if ≥3 months therapy. 3) ADA, CER, ETN, GOL, IFX: CBC every 6 months. 4) RTX: CBC every 3 months. An interactive voice response (IVR) system was employed in outreach. This system sends texts to text-enabled phones and calls to phones that are not text-enabled. The messages state in part: “You are due for a lab test to continue taking your medicine safely. Go to any Kaiser lab within the next week. No appt is needed…” System outcomes analysis was descriptive.
Results: The cohort included 3737 patients, 2365 (63.3%) of whom were taking MTX. Overall, the % of patients due for testing dropped after the intervention began and remained below baseline (Figure). For example, among patients taking MTX during the baseline period, 37% had ≥1 ALT testing gap >100 days (12 weeks + 16 days “grace” period). Comparable proportions of patients had AST, CBC, or Cr testing gaps. During the intervention period, 27% of patients taking MTX had ≥1 ALT testing gap >100 days. Patient and clinician feedback is positive. The outreach requires minimal maintenance. 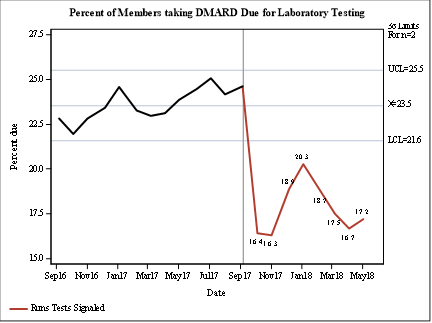
To cite this abstract in AMA style:
Hagen T, Bhardwaja B, Silverman D, Shetterly S, Raebel M. Clinical Laboratory Telephone Communication Outreach to Rheumatology Patients Improves Guideline-Concordant Timeliness of Monitoring of Conventional and Biologic Disease-Modifying Anti-Rheumatic Drugs (DMARDs) [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/clinical-laboratory-telephone-communication-outreach-to-rheumatology-patients-improves-guideline-concordant-timeliness-of-monitoring-of-conventional-and-biologic-disease-modifying-anti-rheumatic-drugs/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-laboratory-telephone-communication-outreach-to-rheumatology-patients-improves-guideline-concordant-timeliness-of-monitoring-of-conventional-and-biologic-disease-modifying-anti-rheumatic-drugs/
