Session Information
Date: Tuesday, October 23, 2018
Title: 5T088 ACR Abstract: RA–DX, Manifestations, & Outcomes IV: CV Co-Morbidities (2814–2819)
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Cardiovascular disease (CVD) represents the leading cause of death in RA, accounting for ~50% of excess mortality. Disease activity, strongly linked to CVD, has been significantly improved by biologic (b) and targeted-synthetic DMARDs in RA. However, the influence of these agents targeting different inflammatory pathways on the CVD risk in RA is not yet clear. We examined the comparative effects of bDMARDs and tofacitinib against conventional synthetic (cs) DMARDs on incident CVD in RA patients.
Methods: RA patients with ≥1 year participation in FORWARD, The National Databank for Rheumatic Diseases, from 1998 through 2017 were assessed for incident CVD (myocardial infarction, stroke and heart failure validated from hospital/death records). DMARDs were categorized into 7 mutually exclusive groups: (1) csDMARDs-referent (2) TNFi (3) Abatacept (4) Rituximab (5) Tocilizumab (6) Anakinra (7) Tofacitinib. For glucocorticoids (GC), a weighted cumulative exposure (WCE) model which combines information about duration, intensity, and timing of exposure into a summary measure was used. Event rates were calculated and compared across bDMARDs using Cox proportional hazards with adjustment for sociodemographics, comorbidities, and RA severity measures.
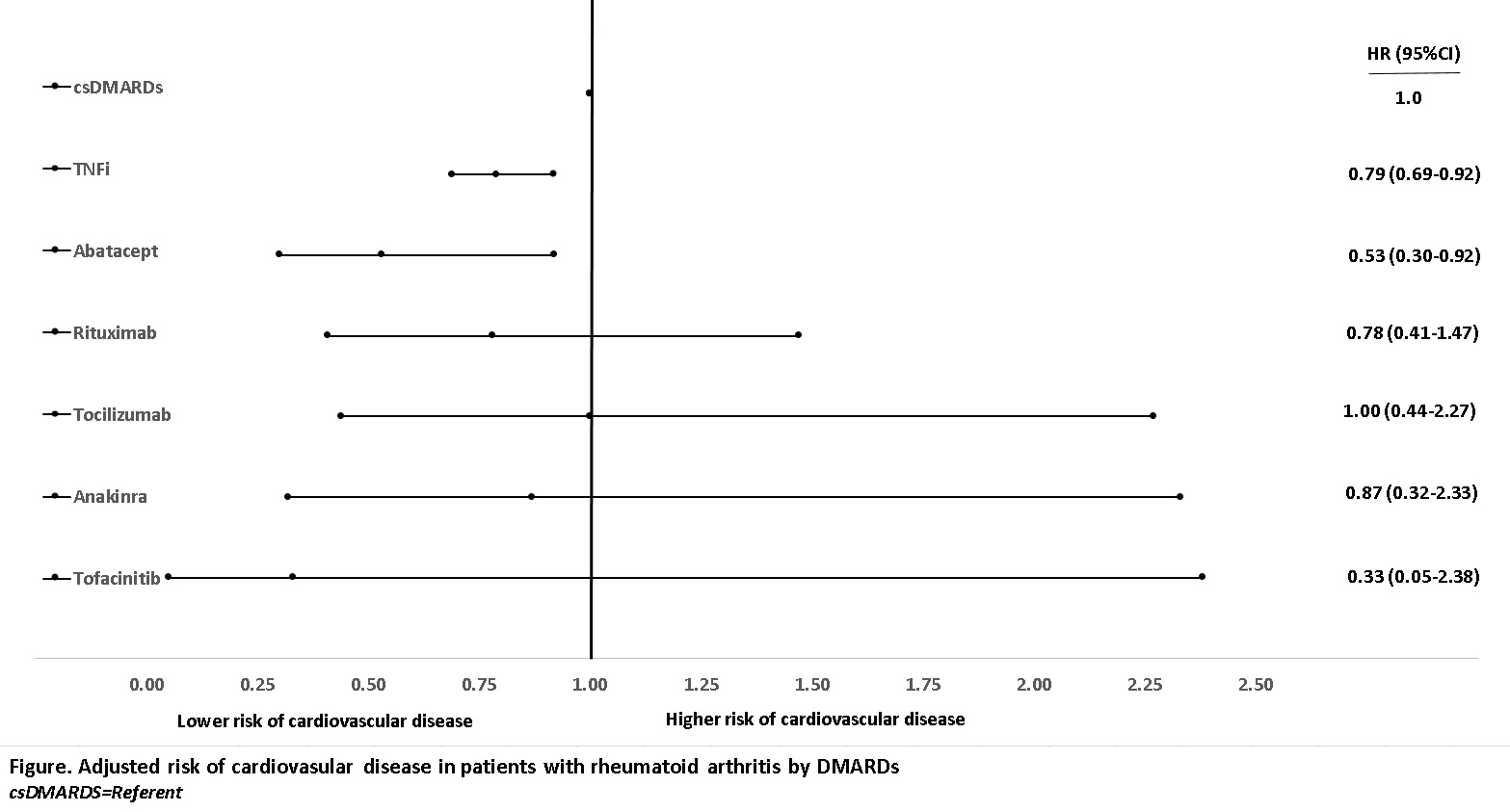
Results: 1,561 CV events were identified in 17,363 RA patients (mean [SD] age 63[13] years, disease duration 18[13] years) during a median (IQR) 4.1 (1.8-8.2) years of followup. TNFi and nonTNFi bDMARDs users had significantly higher disease activity, disability, and comorbidity scores than csDMARD users. The incidence rate (95% CI) of CVD for the entire cohort was 1.78 (1.69-1.87) per 1,000 patient-years. Incidence rates in each DMARD group are shown in the Table. The adjusted model showed a significant CVD risk reduction with TNFi (HR 0.79 [0.69-0.92]) and abatacept (HR 0.53 [0.30-0.92]) compared to csDMARDs whereas GC as WCE was associated with significant risk increase (HR 1.15 [1.11-1.20]). Other bDMARDs and tofacitinib were not significant (Figure). In analysis of individual TNFi, although all TNFi tended to be associated with decreased CV risk, only the risk with infliximab (HR 0.81 [0.67-0.98]) and etanercept (HR 0.78 [0.64-.95]) reached statistical significance.
Conclusion: TNFi, notably infliximab and etanercept, and abatacept were associated with decreased risk of CVD and GC with increased risk. Despite reported similar efficacies of bDMARDs in RA, the difference in CV benefits may be due to drug-specific mechanisms directly influencing the atherosclerosis or metabolic changes.
|
Table. Crude incidence rates and risk of cardiovascular disease in rheumatoid arthritis by treatment |
|||||
|
|
No. of events/exposure |
Patient-years |
Incidence rate (95% CI)* |
Unadjusted HR (95% CI) |
Adjusted HRƪ (95% CI) |
|
All patients |
1,561/17,363 |
87,723 |
1.78 (1.69-1.87) |
– |
– |
|
Glucocorticoids |
646/8,914 |
29,930 |
2.16 (2.00-2.33) |
1.18 (1.15-1.21) |
1.15 (1.11-1.20) |
|
DMARD Category |
|||||
|
csDMARDs (referent) |
1,184/14,418 |
64,265 |
1.84 (1.74-1.95) |
Referent |
Referent |
|
TNFi |
340/7,178 |
20,260 |
1.68 (1.51-1.87) |
0.57 (0.50-0.64) |
0.79 (0.69-0.92) |
|
Abatacept |
15/1,048 |
18,913 |
0.79 (0.48-1.32) |
0.21 (0.13-0.35) |
0.53 (0.30-0.92) |
|
Rituximab |
11/509 |
5,467 |
2.01 (1.11-3.63) |
0.47 (0.26-0.85) |
0.78 (0.41-1.47) |
|
Tocilizumab |
6/390 |
4,411 |
1.36 (0.61-3.03) |
0.31 (0.14-0.69) |
1.00 (0.44-2.27) |
|
Anakinra |
4/137 |
1,419 |
2.82 (1.06-7.51) |
1.08 (0.41-2.90) |
0.87 (0.32-2.33) |
|
Tofacitinib |
1/275 |
1,769 |
0.57 (0.10-4.01) |
0.10 (0.01-0.72) |
0.33 (0.05-2.38) |
|
*Per 1,000 patient-years ƪAdjusted for age, sex, disease duration, socioeconomic status (employment and education level, insurance, location of residency), ethnicity, smoking, hypertension ,diabetes, comorbidity index, BMI, HAQ, NSAIDs, statins, prior count of csDMARDs and bDMARDs, prior CVD history and year of entry |
|||||
To cite this abstract in AMA style:
Ozen G, Pedro S, Michaud K. Cardiovascular Disease Risk with Biologics and Tofacitinib Compared to Conventional Synthetic Dmards in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/cardiovascular-disease-risk-with-biologics-and-tofacitinib-compared-to-conventional-synthetic-dmards-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cardiovascular-disease-risk-with-biologics-and-tofacitinib-compared-to-conventional-synthetic-dmards-in-patients-with-rheumatoid-arthritis/