Session Information
Date: Tuesday, October 23, 2018
Title: Vasculitis Poster III: Immunosuppressive Therapy in Giant Cell Arteritis and Polymyalgia Rheumatica
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
At least 2 biological therapies [tocilizumab (TCZ) and abatacept (ABA)] have been proven to be effective in the management of Giant cell arteritis (GCA) in randomized controlled trials. Nevertheless, their use as steroid sparing agents might need further investigation.
Methods:
We included GCA patients who were treated with TCZ, both intravenous (IV) and subcutaneous (SC), and/or ABA SC (8 mg/kg/month, 162 mg/week, and 125 mg/week respectively). Complete response to the treatment was define as a clinical and serological remission after 12 months of therapy; partial response as clinical or serological remission after 12 months.
Results:
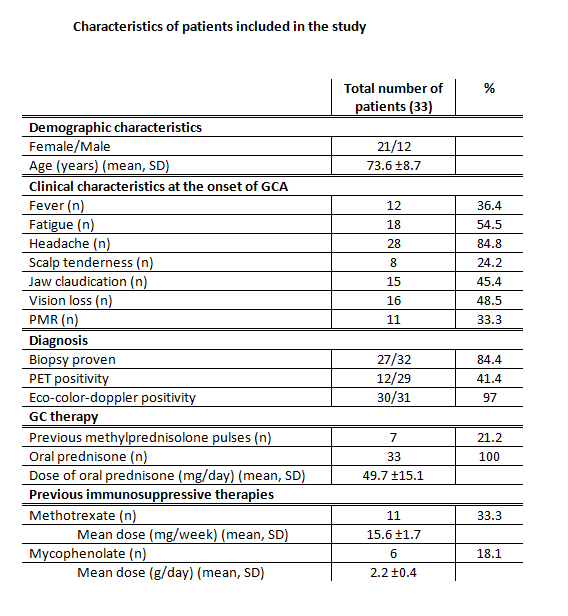
This study included 33 GCA patients [mean age 74, females 63%, mean follow-up 44±34 months). Figure 1 resumes the characteristics of the GCA patients included in the study. Twenty-eight patients out of 33 (85%) received one biologic agent. Five patients (15%) needed a therapeutic switch (one patient from TCZ to ABA, and 4 patients from ABA to TCZ). Patients were treated as follow: 9 with TCZ IV, 11 with TCZ SC, and 18 with ABA. Among the TCZ IV group, all patients experienced a response (57% complete response, and 43% partial response). Among the TCZ SC group, 83% experienced a response (67% complete response, and 16% partial response). Among the ABA group, 86% experienced a response (36% complete response and 50% partial response). After 12 months of therapy, 100% of patients in TCZ
groups, both IV and SC, and 64% of ABA group were treated with low doses of oral prednisone (≤ 7.5 mg/day) as maintenance. We noticed a significant reduction of inflammatory parameters [C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR)] after 12 months of therapy with TCZ [TCZ IV group: mean baseline CRP (mg/dl) 1.9±2.3, mean CRP after 12 months of therapy 0,3±0.2; mean baseline ESR (mm/h) 58.1±25.6, mean ESR after 12 months 9.5±4.2; TCZ SC group: mean baseline CRP 4.5±3.8, mean CRP after 12 months 0.2±0.2; mean baseline ESR 51.9±27, mean ESR after 12 months 6.5±6]. When compared to standard GC regimen, in patients treated with TCZ, both IV and SC, we estimated a median steroid-sparing effect quantifiable in 30 mg/daily in the first month and an overall steroid-sparing effect of 15 mg/daily when assessed in 12 months.
Conclusion:
This study confirms the efficacy of biological therapies in the management of CGA. Besides, in our experience TCZ allowed a significant reduction of GCs use, especially in the first month of therapy, when compared to standard GCs-based regimens.
To cite this abstract in AMA style:
Rossi D, Cecchi I, Rubini E, Radin M, Sciascia S, Roccatello D. Clinical and Serological Outcomes of Patients with Giant Cell Arteritis Treated with Tocilizumab or Abatacept As Steroid-Sparing Agents [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/clinical-and-serological-outcomes-of-patients-with-giant-cell-arteritis-treated-with-tocilizumab-or-abatacept-as-steroid-sparing-agents/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-and-serological-outcomes-of-patients-with-giant-cell-arteritis-treated-with-tocilizumab-or-abatacept-as-steroid-sparing-agents/