Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
A Randomized Phase 3 Trial Evaluating the Safety and Efficacy of Avacopan in Patients with New or Relapsing Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis
Peter A. Merkel1, David Jayne2, Thomas J. Schall3, Pirow Bekker3, Jan Hillson3
1. University of Pennsylvania, Philadelphia PA, USA; 2. University of Cambridge, Cambridge UK; 3. ChemoCentryx Inc., Mountain View CA, USA
Background/Purpose: Anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV) is a potentially life-threatening systemic autoimmune disease characterized by relapses and remissions. Treatment to induce remission usually includes high-dose glucocorticoids in combination with rituximab (RTX) or cyclophosphamide (CYC), followed by less intensive treatment to prevent relapse. The overall burden of disease remains high, with progressive organ system damage and morbidity attributable to therapy. High-dose glucocorticoids are associated with increased risk of infection, metabolic syndrome, bone loss, and an overall negative impact on health-related quality of life (HRQoL). There is a need for less toxic treatment alternatives for AAV. Avacopan (previously CCX168) is a novel, orally bioavailable, highly-selective antagonist of the human C5a receptor that has demonstrated promising efficacy and a favorable safety profile when administered for 12 weeks in Phase 2 studies of patients with active AAV (NCT0136388 and NCT02222155).
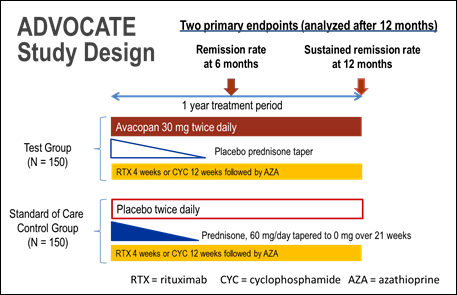
Methods: The ongoing ADVOCATE trial (NCT02994927) is a double-blind, parallel-arm, active-comparator randomized trial comparing avacopan vs. high-dose glucocorticoids in patients (age ≥ 12 years) with AAV, when co-administered with either 1) RTX OR 2) CYC followed by azathioprine or mycophenolate mofetil, for up to 1 year (Figure). The choice of RTX vs CYC is based on investigator judgement. Patients are stratified by: i) intended use of RTX vs CYC, ii) ANCA type (PR3-ANCA vs MPO-ANCA), and iii) newly-diagnosed vs relapsing disease.
The primary trial endpoints are 1) the proportion of patients in remission at week 26 based on BVAS=0 (Birmingham Vasculitis Activity Score) and not taking glucocorticoids; and 2) the proportion of patients who sustain remission from week 26 through week 52. HRQoL will also be assessed in patients through 52 weeks which will add to the understanding of the patient experience in AAV. Other endpoints include adverse events, requirement for rescue medication, markers of renal disease, the Glucocorticoid Toxicity Index, and the Vasculitis Damage Index.
Results: Planned enrollment is ~300 patients from 230 sites in 20 countries, and is expected to be completed in mid-2018. Details of the study design, outcome measures, and baseline patient data will be presented.
Conclusion: The ADVOCATE trial is unique in seeking to advance a nearly glucocorticoid-free induction regimen for AAV and has the potential to have a substantial impact on the approach to treatment of this rare disease.
To cite this abstract in AMA style:
Merkel PA, Jayne D, Schall TJ, Bekker P, Hillson J. A Randomized Phase 3 Trial Evaluating the Safety and Efficacy of Avacopan in Patients with New or Relapsing Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/a-randomized-phase-3-trial-evaluating-the-safety-and-efficacy-of-avacopan-in-patients-with-new-or-relapsing-anti-neutrophil-cytoplasmic-antibody-associated-vasculitis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-randomized-phase-3-trial-evaluating-the-safety-and-efficacy-of-avacopan-in-patients-with-new-or-relapsing-anti-neutrophil-cytoplasmic-antibody-associated-vasculitis/