Session Information
Date: Tuesday, October 23, 2018
Title: Spondyloarthritis Including Psoriatic Arthritis – Clinical Poster III: Treatment
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
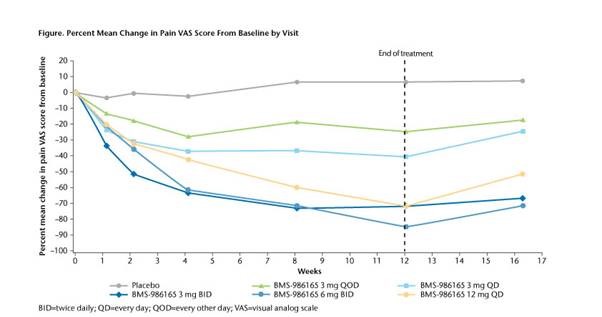
Conclusion: In pts with moderate-to-severe PsO, BMS-986165 demonstrated statistically greater efficacy on skin measures vs pbo at doses ≥3 mg QD and a dose-dependent decrease in pain. BMS-986165 was generally well tolerated. Further evaluation in PsO and psoriatic arthritis is warranted.
| |
||||||
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
||||||
To cite this abstract in AMA style:
Papp KA, Gordon KB, Thaçi D, Morita A, Gooderham M, Foley P, Girgis IG, Kundu S, Banerjee S. Efficacy and Safety of a Potent and Highly Selective Oral Tyrosine Kinase 2 Inhibitor, BMS-986165, in Patients with Moderate-to-Severe Plaque Psoriasis: A Phase II, Randomized, Placebo-Controlled Trial [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-a-potent-and-highly-selective-oral-tyrosine-kinase-2-inhibitor-bms-986165-in-patients-with-moderate-to-severe-plaque-psoriasis-a-phase-ii-randomized-placebo-controlled-tria/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-a-potent-and-highly-selective-oral-tyrosine-kinase-2-inhibitor-bms-986165-in-patients-with-moderate-to-severe-plaque-psoriasis-a-phase-ii-randomized-placebo-controlled-tria/