Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: PROMIS (Patient Reported Outcomes Measurement Information System) has been used in rheumatoid arthritis (RA) patients (Pts) to assess disease activity across multiple domains (i.e. physical function, fatigue, pain interference). AWARE (Comparative and Pragmatic Study of Golimumab IV Versus Infliximab in Rheumatoid Arthritis) is an ongoing Phase 4 study designed to provide a real-world assessment of intravenous Tumor Necrosis Factor inhibitor (TNFi) medications in RA pts. The study utilizes PROMIS and Clinical Disease Activity Index (CDAI) to assess effectiveness. This analysis examined select PROMIS measures to assess (1) relationship between baseline (BL) CDAI and PROMIS-scores, (2) responsiveness of PROMIS after initiation of TNFi and (3) relationship between PROMIS T-scores of the 4 item Profile29v2 Fatigue and Pain Interference domains and respective PROMIS Short Forms (SF).
Methods: AWARE is a prospective, noninterventional, 3-year study at 100 US sites. RA pts were enrolled when initiating TNFi treatment. Treatment decisions are at the discretion of the treating rheumatologist. We report on data from Pts’ BL PROMIS Pain Interference 6b (PI), Fatigue7a (F), Profile29v2 and CDAI. PROMIS T-scores were compared across CDAI disease category (high, moderate etc) using ANOVA. We dichotomized pts based on whether their BL T-score was within 0.5SD of the population mean (i.e. ‘normal’) or not to evaluate for effect modification in the subsequent change in PROMIS T-scores. Data shown are mean ± std dev.
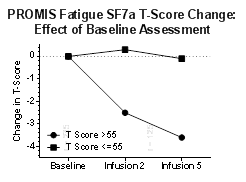
Results: Pts (N=1220) were 59.5± 13.2 yrs, disease duration 8.2 ± 9.9 yrs, 83.4% female, body weight 85.1± 24.2 kg, BMI 31.4 ± 8.51, BL CDAI 32.4 ± 15.6. A significant relationship between PROMIS T-scores (PI, F) and BL CDAI disease activity category was confirmed. There was minimal change in T-score of pts with BL PI and F T-scores </=55 and PF>/=45 over 5 infusions (approx. 5-7 months). Depending on domain, 14.7-27.3% of pts had initial PROMIS T-scores within 0.5SD of normal. There was a significant (p<0.0001) relationship between PI and F T-scores and the respective 4 questions on the P29v2.
Conclusion: Avoiding floor effects in pts who initiated TNFi therapy with near-normal PROMIS scores, PROMIS instruments demonstrated a robust T-score change in response to initiation of TNFi therapy.
To cite this abstract in AMA style:
Bingham III CO, Schwartzman S, Kafka S, Parenti D, Black S, Xu S, Langholff W, Curtis JR. Promis Pain Interference 6b and Fatigue 7a Short Forms and Profile-29 in Rheumatoid Arthritis Patients Treated with TNF Inhibitors [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/promis-pain-interference-6b-and-fatigue-7a-short-forms-and-profile-29-in-rheumatoid-arthritis-patients-treated-with-tnf-inhibitors/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/promis-pain-interference-6b-and-fatigue-7a-short-forms-and-profile-29-in-rheumatoid-arthritis-patients-treated-with-tnf-inhibitors/