Session Information
Date: Monday, October 22, 2018
Title: 4M094 ACR/ARHP Abstract: Health Services Research I: Focus on Big Data (1887–1892)
Session Type: ACR/ARHP Combined Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Biologic DMARDs and tofacitinib account for a large proportion of drug spending in the U.S. Although TNFi drugs have dominated sales, use of other novel agents in RA is increasing. Given the lack of comparative effectiveness studies, understanding patterns around the prescription of second-line drugs is warranted. We used data from the RISE registry to perform a cross-sectional analysis of prevalent prescriptions for biologics and tofacitinib among U.S. rheumatologists.
Methods: RISE is a national, EHR-enabled registry that passively collects data on all patients seen by participating practices, thus reducing the selection bias present in single-insurer claims databases. As of June 2017, RISE held validated data from 663 providers in 110 practices, representing an estimated 19% of the U.S. clinical rheumatology workforce. We identified patients with ≥2 codes for RA ≥30 days apart between July 2016 and June 2017. We tallied the proportion of patients prescribed a TNFi, abatacept, rituximab, tocilizumab, or tofacitinib at least once during this period, overall, by region, and by practice. In this cross-sectional analysis of prevalent prescriptions, each patient was included only once; i.e., patients with >1 of these drugs were assigned to the first drug prescribed during the study period. To reduce variability in practice-level estimates, practices with < 30 RA patients (23 of the 110 practices) were excluded.
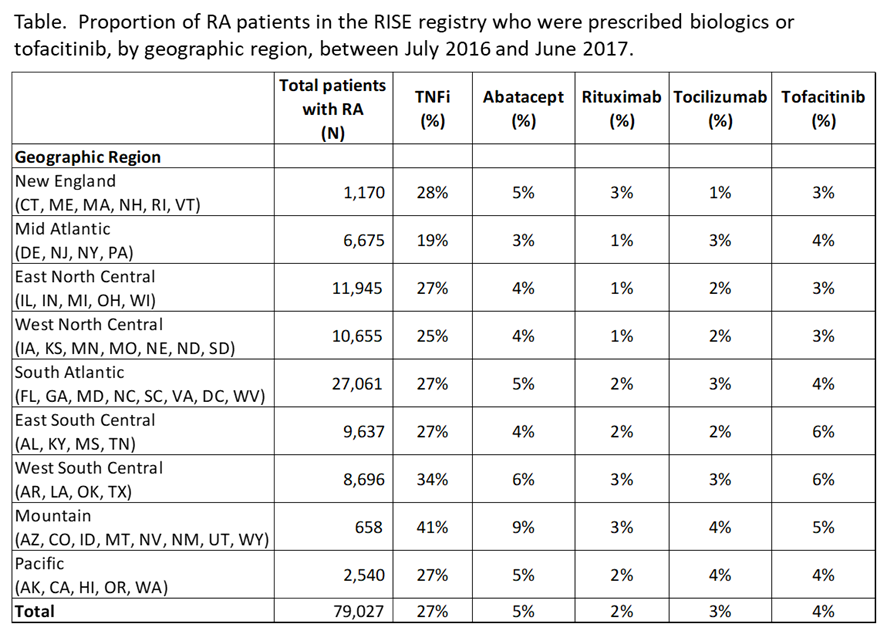
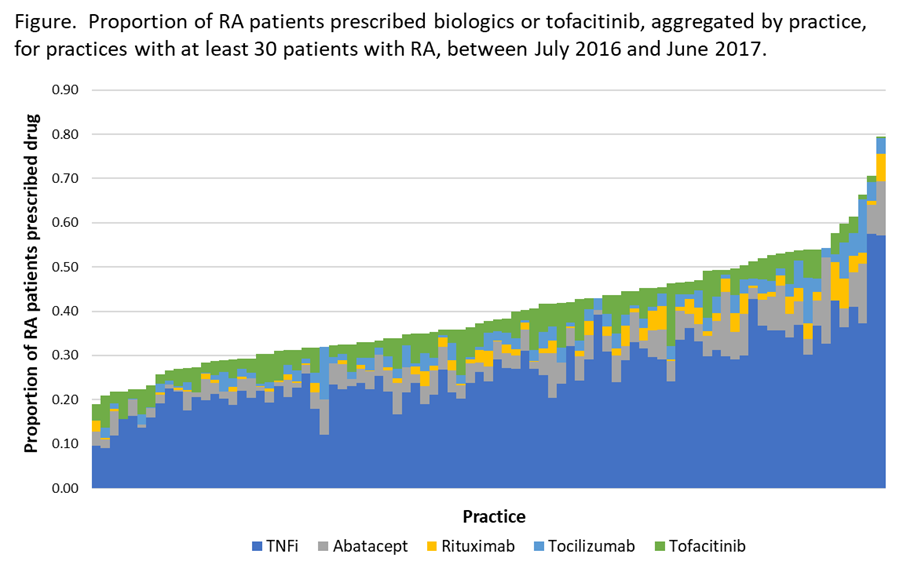
Results: We included 79,027 patients from 87 practices. Overall, 40% of patients were prescribed any biologic or tofacitinib during the study period. Besides TNFi, abatacept was most commonly prescribed (4.7% of patients), followed by tofacitinib (4.2%), tocilizumab (2.7%), and rituximab (1.9%). Patterns were similar across regions (Table), but there was wide practice-level variation (Figure): For example, among practices that prescribed any abatacept (N=85), abatacept represented 2-36% of all biologics prescribed for RA within that practice. Similar patterns were seen for tocilizumab (N=83, range 1-38%), and tofacitinib (N=82, range 2-35%).
Conclusion: We found significant practice-level variation in prescriptions for newer DMARDs for RA, especially for non-TNFi biologics and tofacitinib. Future studies should assess differences in outcomes and the role of payers among populations receiving different treatments.
Disclaimer: This data was supported by the ACR’s RISE Registry. However, the views expressed represent those of the authors, not necessarily those of the ACR.
To cite this abstract in AMA style:
Schmajuk G, Evans M, Kay J, Clowse MEB, Morgan E, Reimold A, Johansson T, Lewis L, Yazdany J. Practice Variation in Prescriptions of Non-TNFi Biologics and Tofacitinib: Data from the Rheumatology Informatics System for Effectiveness (RISE) Registry [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/practice-variation-in-prescriptions-of-non-tnfi-biologics-and-tofacitinib-data-from-the-rheumatology-informatics-system-for-effectiveness-rise-registry/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/practice-variation-in-prescriptions-of-non-tnfi-biologics-and-tofacitinib-data-from-the-rheumatology-informatics-system-for-effectiveness-rise-registry/