Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The use of immunosuppressant (IS) with glucocorticoid is recommended as remission induction treatment for severe cases with antineutrophil cytoplasmic antibody (ANCA) associated vasculitis (AAV). However, we sometimes experience the discontinuation of IS due to its adverse event. We examined the clinical characteristics of patients with AAV who received IS treatment to identify the risk factors of the discontinuation of IS.
Methods: We retrospectively analyzed the clinical data of patients with AAV from 2005 to 2016. The clinical data included patients-demographics, the use of IS, the adverse event of IS, the cumulative dose of glucocorticoid until the start of IS, and the continuity of IS. The definition of discontinuation of IS was switch to glucocorticoid-single therapy, switch to another IS, or delay of IS re-administration due to the adverse event of IS. First IS used for remission induction treatment was analyzed in each patient. Activity of daily living was assessed by performance status (PS) proposed by Eastern Cooperative Oncology Group. The survival of IS use was estimated by Kaplan-Meier method, and the difference between the survival curves was statistically analyzed by log rank analysis.
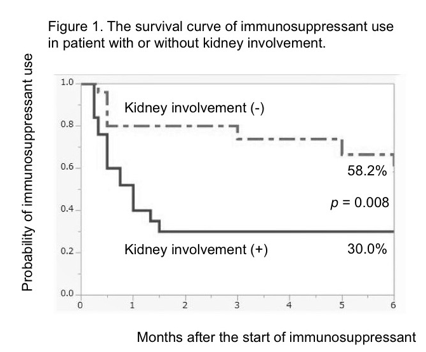
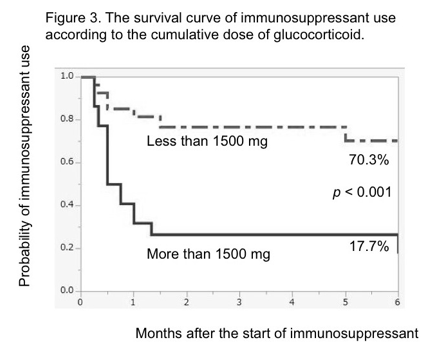
Results: We found 162 patients with AAV, and 50 patients were treated with IS for remission induction treatment. Among 50 patients, 26 patients experienced the discontinuation of IS due to its adverse event. The use of IS were as follows: intravenous cyclophosphamide 34, oral cyclophosphamide 11, methotrexate 4, and cyclosporine 1. Kaplan Meier analysis showed significant differences in the survival rate of IS use between patients with or without kidney involvement (30.0% vs. 58.2%, p = 0.008, Figure 1), between PS scores with 0 to 2 and 3 to 4 (48.8% vs. 20.0%, p = 0.029, Figure 2), and between the cumulative doses of glucocorticoid of less and more than 1500 mg (70.3% vs. 17.7%, p <0.001, Figure 3) at 6 months.
Conclusion: This study showed that kidney involvement, poor PS, higher cumulative dose of glucocorticoid until the start of IS were the risk factors of the discontinuation of IS. We suggest that physicians should pay attention to these factors to reduce the risk of discontinuation of IS when treating patients with AAV using IS.
To cite this abstract in AMA style:
Murosaki T, Sato T, Nagatani K, Minota S. Kidney Involvement, Poor Performance Status, and Higher Cumulative Dose of Glucocorticoid Are the Risk Factors of the Discontinuation of Immunosuppressant in Patients with Antineutrophil Cytoplasmic Antibody Associated Vasculitis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/kidney-involvement-poor-performance-status-and-higher-cumulative-dose-of-glucocorticoid-are-the-risk-factors-of-the-discontinuation-of-immunosuppressant-in-patients-with-antineutrophil-cytoplasmic-a/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/kidney-involvement-poor-performance-status-and-higher-cumulative-dose-of-glucocorticoid-are-the-risk-factors-of-the-discontinuation-of-immunosuppressant-in-patients-with-antineutrophil-cytoplasmic-a/