Session Information
Date: Monday, October 22, 2018
Title: Rheumatoid Arthritis – Treatments Poster II: PROs, Safety and Comorbidity
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
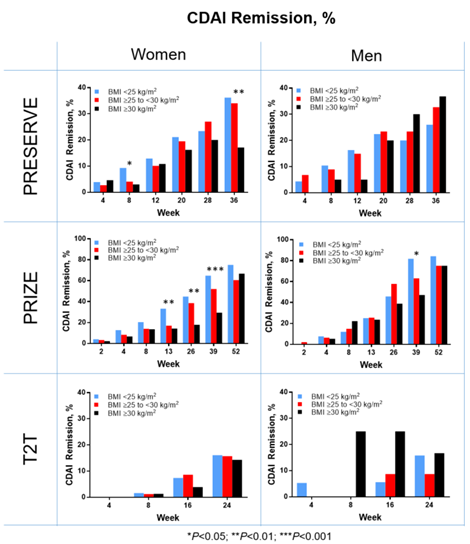
Background/Purpose: In patients with RA treated with TNF-α inhibitors, a higher BMI has been associated with lower odds of achieving disease remission. We evaluated the effect of BMI on response to etanercept (ETN) treatment in women and men with RA, (a) during open-label treatment and (b) following dose reduction or dosing off in patients who achieved low disease activity (LDA) or remission.
Methods: In this post hoc analysis, data were collected from three randomized trials (PRESERVE, N=834; PRIZE, N=306; T2T, N=489) in which patients with RA were assigned to an open-label treatment with ETN (50 mg) and MTX for 24-52 weeks, followed by randomized double-blind treatment with ETN (25 mg or 50 mg) + MTX, placebo + MTX, or placebo for 28-52 weeks in those who achieved LDA or remission. Observed cases data were analyzed by BMI (<25 kg/m2, ≥25 and <30 kg/m2, or ≥30 kg/m2) for women and men separately, using a one-way analysis of covariance model for continuous variables and a logistic regression model for categorical variables. The outcomes included changes from baseline in Clinical Disease Activity Index (CDAI), 28-joint DAS with CRP level (DAS28 CRP) or ESR (DAS28 ESR), and HAQ – Disability Index (HAQ-DI), as well as the percentages of patients who achieved CDAI remission (CDAI ≤2.8) or LDA (CDAI >2.8 and ≤10). The analysis was sponsored by Pfizer Inc. Medical writing assistance was provided by Vojislav Pejović of Engage Scientific Services.
Results: In the open label periods of all three studies, there was no significant BMI effect on treatment response to ETN in male patients, except for CDAI remission at a single visit in PRIZE (Figure). In open-label periods of the PRIZE and T2T trials, a significantly smaller decrease in DAS28 CRP and DAS28 ESR in women with BMI ≥30 kg/m2, compared with the other two BMI categories, was observed at most visits. In addition, in PRIZE trial (but not in PRESERVE or T2T), women, but not men, with BMI ≥30 kg/m2 had a significantly smaller decrease in CDAI and HAQ-DI scores and lower rates of CDAI LDA at most visits, compared with their counterparts with BMI <30 kg/m2. For CDA remission, there was evidence of the effect of BMI ≥30 kg/m2 in women in both PRIZE and PRESERVE (Figure). Overall, these nominally significant differences between women with BMI ≥30 kg/m2 and <30 kg/m2 were transient: most of them diminished or were no longer significant toward the end of the open-label periods. In randomized, double-blind periods, there were no discernible trends attributable to BMI category in either women or men.
Conclusion: Results of this post hoc analysis suggest that, in men with RA, there was no impact of BMI on ETN efficacy. In women, there was evidence of a transient negative impact of BMI ≥30 kg/m2 on ETN efficacy in open-label periods, but it diminished by the end of the induction period and was not consistent across trials. There was no evidence of BMI effect in men or women during the double-blind periods.
To cite this abstract in AMA style:
Alten R, Mysler E, Wajdula A, Jones H, Pedersen R, Marshall L. Efficacy of Etanercept By Body Mass Index in Women and Men with Rheumatoid Arthritis: A Post Hoc Analysis of Three Randomized Trials [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-etanercept-by-body-mass-index-in-women-and-men-with-rheumatoid-arthritis-a-post-hoc-analysis-of-three-randomized-trials/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-etanercept-by-body-mass-index-in-women-and-men-with-rheumatoid-arthritis-a-post-hoc-analysis-of-three-randomized-trials/