Session Information
Date: Monday, October 22, 2018
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Hyperimmunoglobulinemia D syndrome (HIDS), also known as mevalonate kinase deficiency (MKD), and TNF receptor-associated periodic syndrome (TRAPS) are rare auto-inflammatory diseases grouped as periodic fever syndromes. There is limited evidence on treatment outcomes for these syndromes. We assessed the efficacy/effectiveness and safety of current treatments used for HIDS and TRAPS.
Methods: A systematic literature review was conducted using Embase®, MEDLINE®, MEDLINE®-In Process and Cochrane library to identify randomized controlled trials (RCTs) and real-world (prospective and retrospective) studies of HIDS/TRAPS patients (pts) published in English language as full-text articles (2000 to September 2017) or conference abstracts (2014 to September 2017). Studies with <5 pts were excluded.
Results: Of the 3342 retrieved publications, 27 studies were included (11 HIDS, 9 TRAPS, and 7 had both cohorts). The majority of studies were full-text (20), published after 2010 (21), and retrospective (13). Studies included two RCTs [one for canakinumab (CAN) vs placebo (PBO); one for simvastatin (SIM) vs PBO]. HIDS pts were most often treated with anakinra (ANA; 9), CAN (7), and corticosteroids (CST; 5); and TRAPS pts with etanercept (ETA; 10), CAN (6), ANA (6), and CST (5).
In the CAN RCT, at 16 weeks (wks), a significantly higher proportion of CAN treated pts vs PBO achieved clinical response ( i.e., resolution of the index flare at Day 15 and no new disease flare over 16 wks) in both cohorts (HIDS: 35% vs 6%; TRAPS: 45% vs 8%). The clinical response was higher with CAN vs PBO up to 40 wks. A crossover RCT of SIM in HIDS pts showed a decrease in urine mevalonic acid levels in 100% of pts at 24 wks and reduced the number of febrile days in 83% of pts.
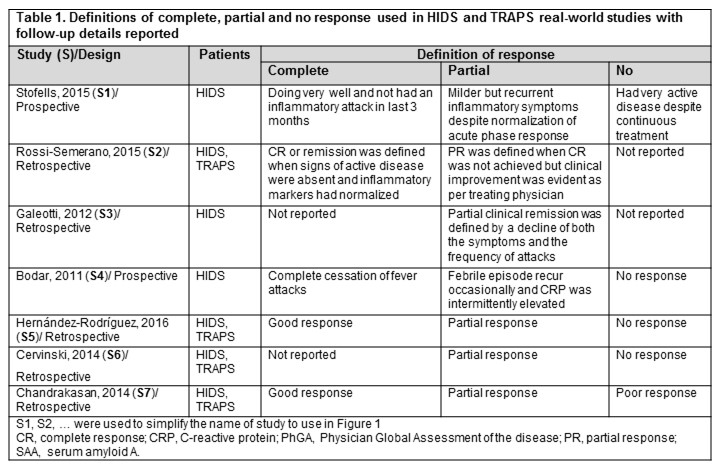
In real-world studies, complete (CR) or partial response (PR) was often assessed, and their definitions and follow-ups (FUP) varied (Table 1; Figure 1). At mid-term FUP, in HIDS pts, CR was 50% [for tocilizumab (TOC)] and 11%-30% (ANA); in TRAPS pts, CR was 100% (CAN) and 33% (ANA). At long-term FUP, in HIDS pts, CR was 100% (ANA), 100% [colchicine (COL) + prednisone (PRD)] and 50% (CAN); in TRAPS pts, CR was 100% (CAN), 100% (COL + PRD), 50% (ETA) and 33% (ANA). CAN, TOC, SIM, ETA and ANA were generally well-tolerated by HIDS and TRAPS pts; but for ETA and ANA, local injections site reactions were commonly reported.
Conclusion: CAN was efficacious in controlling and preventing flares in HIDS and TRAPS pts in the studies assessed. Although response criteria and treatments were not directly comparable, the available evidence suggested higher effectiveness of CAN in the real-world setting.
To cite this abstract in AMA style:
Kuemmerle-Deschner JB, Gautam R, George AT, Raza S, Lomax KG, Hur P. A Systematic Literature Review of Efficacy and Safety of Current Therapies for the Treatment of Hyperimmunoglobulinemia D Syndrome and TNF Receptor-Associated Periodic Syndrome [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/a-systematic-literature-review-of-efficacy-and-safety-of-current-therapies-for-the-treatment-of-hyperimmunoglobulinemia-d-syndrome-and-tnf-receptor-associated-periodic-syndrome/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-systematic-literature-review-of-efficacy-and-safety-of-current-therapies-for-the-treatment-of-hyperimmunoglobulinemia-d-syndrome-and-tnf-receptor-associated-periodic-syndrome/