Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: To prevent disease progression, treat-to-target recommendations for rheumatoid arthritis (RA) include the evaluation and adjustment of drug therapy at least every 3 months until a target level of remission or low disease activity is achieved. It is well established that anti-tumor necrosis factor (TNF) treatment modifies associations between disease activity and radiographic progression over periods of 1-2 years.1 We aimed to assess whether TNF inhibition modifies this association over the initial 3-6 months of treatment for early RA.
Methods: Methotrexate (MTX)-naïve early RA patients randomized to double-blind treatment with adalimumab (ADA)+MTX combination therapy vs. MTX monotherapy were drawn from the Phase III PREMIER clinical trial. Associations between week 12 disease activity, assessed using DAS28-CRP(4), and changes in modified total sharp score (mTSS) from baseline to week 26 were compared between the ADA+MTX and MTX arms using generalized additive regression. mTSS progression was modeled as an absolute score change and as a worsening of ≥5 units.2 Models were fit with and without adjustment for baseline mTSS, DAS28, numbers of tender and swollen joints, and RA duration.
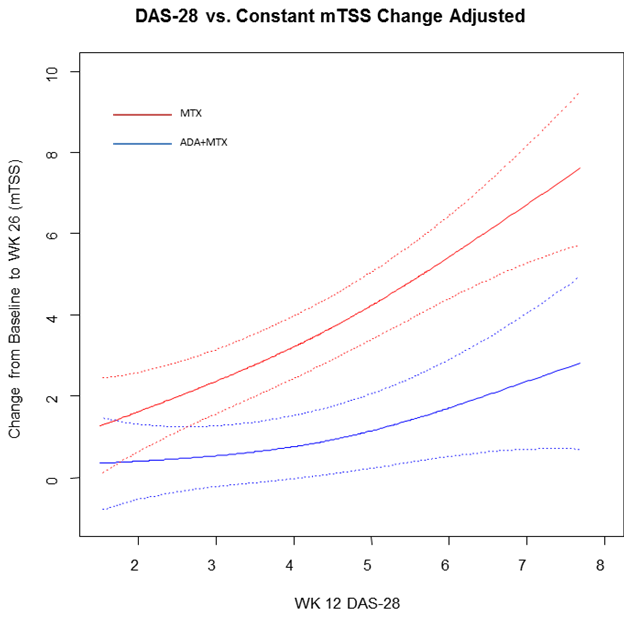
Results: A total of 219 MTX-treated patients and 241 ADA+MTX-treated patients were included in the investigation. The mean age was 52 years, 73% were female, and the average RA duration was 9 months. In the adjusted analyses, patients treated with ADA+MTX experienced limited mean increases in mTSS from baseline to week 26 that were numerically similar across all levels of week 12 DAS28, whereas patients treated with MTX showed a sharp increase in mTSS progression with increasing week 12 DAS28 (Figure). Compared to patients who were treated with MTX alone, those treated with ADA+MTX achieving a DAS28 £2.6 at week 12 had a significantly smaller change in mTSS (difference: -1.59; 95% CI: -2.74, -0.44) and a significantly lower risk of mTSS progression of ≥5 units (odds ratio [OR]: 0.19; 95% CI: 0.06, 0.61) at week 26. Results were similar for unadjusted analyses.
Conclusion: Early RA patients achieving a given disease activity level by week 12 with ADA+MTX experience less radiographic progression by week 26 than those achieving the same disease activity level with MTX alone. This could reflect the slower onset of response to MTX and/or a separate structure-protective effect for anti-TNF treatment. These aspects should be considered when setting disease activity targets with the goal of limiting radiographic progression.
References:
1. Emery P et al. Journal of Rheumatology. 2009; 36(6): 1429-1441.
2. Bruynesteyn et al. Arthritis and Rheumatism. 2002; 46(4): 913-920.
Disclosure:
R. F. van Vollenhoven,
Abbott, BMS, GSK, HGS, MSD, Pfizer, Roche, UCB,
2,
Abbott, BMS, GSK, HGS, MSD, Pfizer, Roche, UCB,
5;
J. W. Shaw,
Abbott Laboratories,
3;
M. A. Cifaldi,
Abbott Laboratories,
3,
Abbott Laboratories,
1;
J. Signorovitch,
Abbott Laboratories,
5;
E. Q. Wu,
Analysis Group, Inc,
3;
T. Samuelson,
Analysis Group,
3;
E. Faust,
Analysis Group,
3;
P. Emery,
Abbott Laboratories,
5.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/differences-in-short-term-radiographic-progression-following-early-response-to-adalimumab-plus-methotrexate-vs-methotrexate-alone/