Session Information
Date: Sunday, October 21, 2018
Title: Rheumatoid Arthritis – Treatments Poster I: Strategy and Epidemiology
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: What is the optimal glucocorticoid (GC) bridging therapy with MTX monotherapy in early arthritis?
Methods: In trial A, early RA and UA (arthritis in ≥1 joint(s), <1 year symptoms) patients were randomised to 3 different treatment arms. Here we used data of arm C where patients were treated with prednisone (15 mg/day, tapered to 0 in 10 weeks) and MTX (25mg/week).
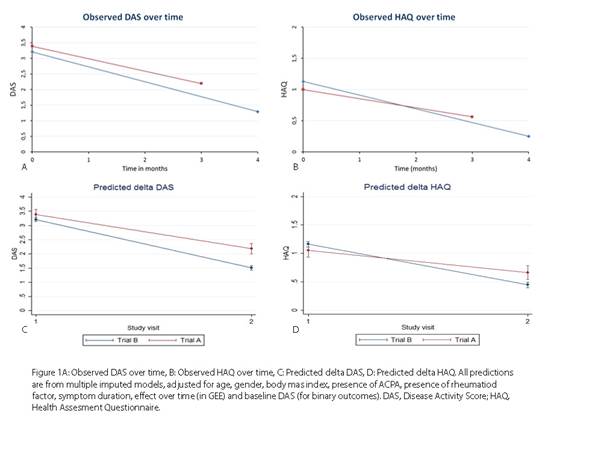
In trial B, RA and UA (arthritis in ≥1 joint and ≥1 other painful joint) patients were treated with prednisone (60 mg/day, tapered in 7 weeks to 7.5 mg/day, continued to 4 months) and MTX (25 mg/week). We compared changes in DAS and HAQ and percentages with DAS≤2.4 and with DAS<1.6 at first evaluation (3 months in trial A, 4 months in trial B). After multivariate normal imputation we applied generalized estimating equations (GEE) for linear outcomes and logistic regression models for binary outcomes, adjusted for potential baseline confounders (figure 1). Adverse events were compared using binominal probability test.
Results: At baseline, patients in trial A (n=97) were more often APCA positive (77% vs 56%) and less often had UA (2% vs 20%) than in trial B (n=610). Baseline DAS, HAQ and symptom duration were comparable.
At the first evaluation time point (median 3.0 (IQR 2.98-3.2) months in trial A, 4.0 (3.8-4.2) in trial B), DAS and HAQ had decreased significantly less in trial A (DAS β 0.500 (95% CI 0.276; 0.725), and HAQ 0.330 (0.189; 0.470) (figure 1).
Fewer patients in trial A achieved DAS<1.6 (29% vs 63%) (adjusted OR 0.215 (95% CI 0.124; 0.373) and DAS≤2.4 (56% vs 81% (adjusted OR 0.249 (0.143; 0.435)). Presence of ACPA was positively associated with achieving DAS<1.6 in trial B, but not in trial A. Reported serious adverse events were 23 per 100 patient years in trial A and 8 in trial B (table 1).
Table 1. Most frequently reported adverse events, per 100 patient years
|
|
Trial A |
Trial B (n=610) |
p |
|
Malaise |
42.9 |
9.45 |
0.000 |
|
Gastrointestinal symptoms |
97.6 |
48.7 |
0.004 |
|
Hypertension |
0.00 |
9.95 |
0.091 |
|
Hyperglycaemia (>7.8mmol/l) |
0.00 |
8.46 |
0.130 |
|
Infections |
46.9 |
39.8 |
0.582 |
|
Skin rashes |
27.3 |
7.96 |
0.014 |
|
Hair loss |
23.4 |
9.45 |
0.074 |
|
Headache |
31.2 |
8.95 |
0.008 |
|
Depression/feeling sad |
31.2 |
11.9 |
0.031 |
|
Bone marrow depression |
15.6 |
0.00 |
0.000 |
|
High creatinine (above normal) |
11.7 |
0.00 |
0.001 |
|
Liver enzymes (above normal) |
35.1 |
22.4 |
0.229 |
|
Dizziness |
3.90 |
5.47 |
0.837 |
|
Dyspnoea |
0.00 |
3.48 |
0.432 |
Conclusion: In early arthritis patients, MTX with prednisone 60 mg/day tapered in 7 weeks to and continued at 7.5 mg/day was associated with better early clinical outcomes and fewer (serious) adverse events although slightly more hypertension and hyperglycaemia than MTX with prednisone 15mg daily tapered to nil in 10 weeks.
To cite this abstract in AMA style:
Brilman EG, van der Pol JA, de Jong PH, Weel AE, Hazes J, Huizinga TWJ, Allaart CF. Corticosteroid Bridging Strategies with Methotrexate Monotherapy in Early Rheumatoid and Undifferentiated Arthritis; A Comparison of Efficacy and Toxicity in 2 Clinical Trials [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/corticosteroid-bridging-strategies-with-methotrexate-monotherapy-in-early-rheumatoid-and-undifferentiated-arthritis-a-comparison-of-efficacy-and-toxicity-in-2-clinical-trials/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/corticosteroid-bridging-strategies-with-methotrexate-monotherapy-in-early-rheumatoid-and-undifferentiated-arthritis-a-comparison-of-efficacy-and-toxicity-in-2-clinical-trials/