Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: The routine assessment of patient index data 3 (RAPID3) was recently designed as a pooled index of 3 patient-reported outcomes (PROs): physical function, pain and patient global estimate. Compared to other simplified disease activity scores RAPID3 may be more desirable in a clinical setting due to the shorter scoring time required. The study objective was to assess in a Canadian real-world setting the RAPID3 outcomes in rheumatoid arthritis (RA) patients treated with infliximab (IFX).
Methods: BioTRAC is an ongoing Canadian registry of RA, AS or PsA patients initiating treatment with IFX or golimumab (GOL) as first biologics or after having been treated with a biologic < 6 months. A total of 806 RA patients initiated IFX between 2002 and 2012 and were included in this analysis. RAPID3 was assessed both in continuous and categorical scales defining high activity (>12), moderate activity (6.1-12), low activity (3.1-6), and remission (≤3).
Results: Mean (SD) age of the patient cohort was 55.3 (13.5) yrs, and mean (SD) duration since diagnosis was 8.9 (9.3) yrs. Mean (SD) patient characteristics at baseline were: ESR = 32.5 (24.2) mm/hr; CRP = 19.0 (24.0) mg/L; SJC-28 = 10.8 (7.0); TJC-28 = 12.7 (8.0); HAQ-DI = 1.7 (0.7); DAS28-CRP = 5.4 (1.3); Pain-VAS= 5.8 (2.4); PGA-VAS = 6.6 (2.1); SGA-VAS = 6.1 (2.4) and CDAI = 36.4 (16.2). By 6 months of treatment, clinically meaningful and statistically significant (P<0.05) improvements were observed in all parameters which were sustained over 24 months. Similarly, the mean (SD) RAPID3 score significantly decreased from 17.3 (6.2) at baseline to 11.4 (6.9), 10.5 (7.0), and 9.3 (6.9) at 6, 12, and 24 months, respectively. Figure 1 shows the RAPID3 disease categories over time upon IFX treatment. The proportion of patients with high disease activity decreased from 80.6% at baseline to 34.2% at month 24. Furthermore, the proportion of patients with low activity or remission increased from 6.0% at baseline to 41.6% at month 24. Figure 1: RAPID3 Disease Activity Categories Over Time
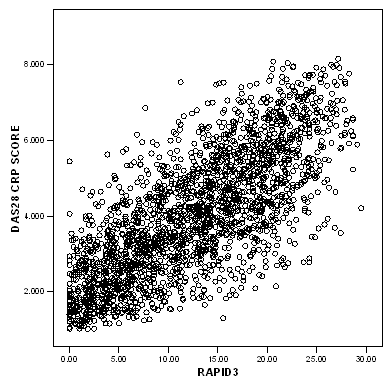
The positive correlation over time between DAS28-CRP and RAPID3 (rho=0.748; P<0.001) and between SDAI and RAPID3 (rho=0.743; P<0.001) further confirms the validity of the RAPID3 as disease activity score in real-life RA patients. Figure 2: Correlation between RAPID3 and DAS28-CRP Over Time
Conclusion: The results of this Canadian real-world observational study demonstrate that, over 2 yrs of treatment, IFX is effective in reducing symptom severity and improving patient-reported outcomes in RA patients. Furthermore, the data from this registry confirmed the validity of the RAPID3 index as disease activity measure in a real-world RA cohort.
Disclosure:
A. Chow,
None;
M. M. Khraishi,
None;
J. F. Rodrigues,
None;
S. M. Otawa,
Janssen Canada Inc,
3;
H. Khalil,
Janssen Canada Inc,
3.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-effectiveness-of-infliximab-in-improving-routine-assessment-of-patient-index-data-3-outcomes-the-canadian-experience/