Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Belimumab, an inhibitor of B lymphocyte stimulator, is approved in adults with active, autoantibody-positive systemic lupus erythematosus (SLE) receiving standard of care. There is limited information on all-cause healthcare resource utilization (HCRU) and costs before/after initiation of belimumab treatment. Data from privately-insured US administrative claims databases can provide valuable descriptive information on HCRU and costs under routine care settings.
Methods: A retrospective analysis (study 206345) was conducted using the Truven Health MarketScan® Commercial Claims and Encounters database (Sept 01, 2010 to Dec 31, 2015). Patients were 18–64 years of age with a SLE diagnosis (ICD-9: 710.0 or ICD-10: M32) and ≥1 belimumab infusion. The index date was the date of the first belimumab infusion. Continuous enrollment was required 6 months pre‑index and ≥3 post‑index date. Here, we present data from patients with 6 months of post‑index follow-up.
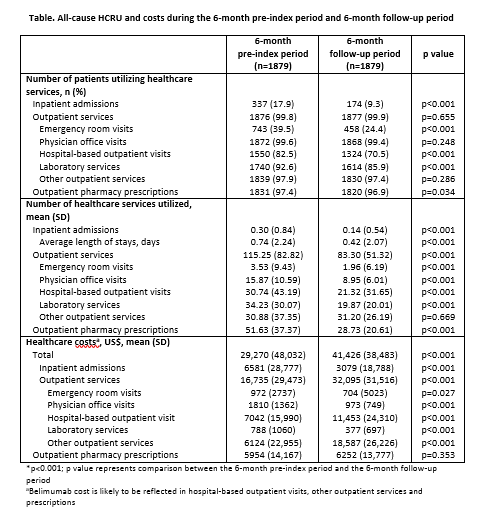
Results: This analysis comprised 1879 patients with 6 months of post-index follow-up. The proportion of patients utilizing healthcare services was significantly lower after belimumab initiation versus the pre-index period for inpatient admissions, and for some outpatient services such as emergency room visits, hospital-based outpatient visits and laboratory services (all p<0.001; Table). The number of healthcare services utilized was also significantly lower after initiation versus the pre-index period for inpatient admissions and several outpatient services, including emergency room visits, physician office visits, hospital-based outpatient visits, laboratory services and outpatient pharmacy prescriptions (all p<0.001). Total all-cause healthcare costs were significantly higher following belimumab initiation, compared with the pre-index period (p<0.001). Outpatient service costs, including hospital-based outpatient visits and other outpatient costs, were significantly higher following initiation (all p<0.001). However, inpatient admissions, physician office visits and laboratory costs were significantly lower after initiation (all p<0.001).
Conclusion: This study provides valuable real-world information about HCRU and costs before and after initiation of intravenous belimumab in a large sample of US patients with SLE. While total costs increased in the 6-month follow-up period, in part due to the cost of belimumab, significant reductions in inpatient admissions, emergency room visits and physician office visits, as well as the costs associated with these services were observed. The effect of belimumab treatment on HCRU and costs, particularly beyond the first 6 months, requires further exploration.
Study funded/conducted by GSK. Editorial assistance provided by Jennie McLean, PhD, of Fishawack Indicia Ltd, UK, funded by GSK
To cite this abstract in AMA style:
Bell CF, Priest J, Stott-Miller M, Kan H, Amelio J, Song X, Limone B, Noxon V, Costenbader KH. Healthcare Resource Utilization and Costs in Patients with Systemic Lupus Erythematosus before and after Initiating Treatment with Intravenous Belimumab: A US Claims Database Analysis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/healthcare-resource-utilization-and-costs-in-patients-with-systemic-lupus-erythematosus-before-and-after-initiating-treatment-with-intravenous-belimumab-a-us-claims-database-analysis/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/healthcare-resource-utilization-and-costs-in-patients-with-systemic-lupus-erythematosus-before-and-after-initiating-treatment-with-intravenous-belimumab-a-us-claims-database-analysis/