Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Anifrolumab is a fully human anti–interferon-ɑ receptor 1 monoclonal antibody in Phase III development as an intravenous (IV) therapeutic for systemic lupus erythematosus (SLE). In Phase IIb trials, IV anifrolumab (300 mg every 4 weeks) significantly decreased SLE disease activity and was well-tolerated.1 In this Phase I, blinded, randomized, controlled study (NCT02601625), we profiled the pharmacokinetics (PK), safety, and tolerability of anifrolumab administered subcutaneously (SC) and IV to healthy volunteers.
Methods: Thirty male and female adults were assigned to three sequential treatment cohorts of equal size (anifrolumab 300 mg SC injection, anifrolumab 300 mg IV, anifrolumab 600 mg SC by infusion). Individuals were randomized within each cohort to receive a single dose of either anifrolumab (n=6/cohort) or placebo (PBO) (n=4/cohort). Serial blood samples were collected up to Day 84. Serum anifrolumab concentrations were analyzed with a validated assay. PK parameters were estimated by noncompartmental analysis. Immunogenicity of anifrolumab was assessed by measuring serum anti-drug antibodies (ADAs).
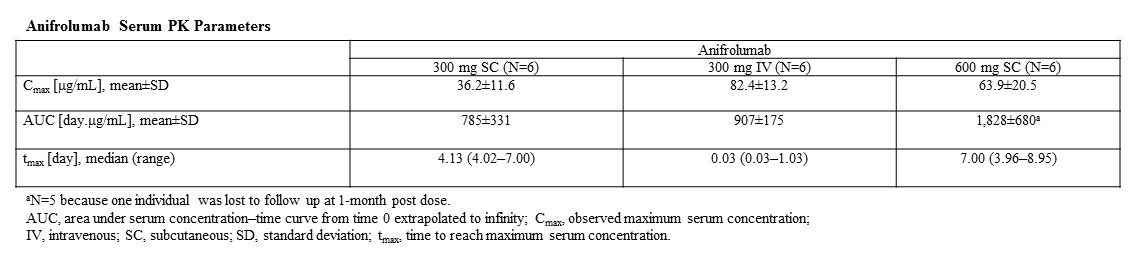
Results: Anifrolumab serum concentration–time profiles and PK parameters in healthy volunteers are presented in the figure and table, respectively. Anifrolumab serum concentrations were below the limit of detection in all individuals by 84 days post dose. Maximum serum concentrations in the SC cohorts occurred after 4–7 days. Exposure to SC anifrolumab increased approximately dose proportionally from 300 mg to 600 mg based on AUC. Anifrolumab exposure after SC administration of the 300-mg dose reached approximately 87% of the IV administration exposure. SC administration of anifrolumab 300 mg and PBO elicited minimal injection-site reactions. Transient injection-site induration occurred in five of six individuals in the anifrolumab 600-mg group and two of four in the PBO group. Transient, mild to moderate injection-site induration and pruritus occurred simultaneously in two of six individuals in the anifrolumab 600-mg group, but not in those in the PBO group. Adverse events were reported by 50% (n=9) of anifrolumab-treated and 33% (n=4) of PBO-treated individuals. No serious adverse events were observed. ADAs were detected in only one individual in the anifrolumab 300-mg IV group at the Day-84 assessment.
Conclusion: Exposure of anifrolumab 300 mg SC was approximately 87% of IV administration, with single SC administrations of anifrolumab being generally well-tolerated in healthy volunteers.
Reference:
1. Furie R, et al. Arthritis Rheumatol. 2017;69:376–86.
To cite this abstract in AMA style:
Tummala R, Rouse T, Berglind A, Santiago L. Safety, Tolerability, and Pharmacokinetics of Subcutaneous and Intravenous Anifrolumab in Healthy Volunteers [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/safety-tolerability-and-pharmacokinetics-of-subcutaneous-and-intravenous-anifrolumab-in-healthy-volunteers/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-tolerability-and-pharmacokinetics-of-subcutaneous-and-intravenous-anifrolumab-in-healthy-volunteers/