Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Blinded transitioning from originator infliximab (INX) to biosimilar CT-P13 was not inferior to continuing INX treatment.1 Open label mandatory transitioning resulted in a slightly lower 1-year CT-P13 retention rate than for INX in a historical cohort.2 However, non-mandatory transitioning might be preferred above mandatory. Therefore, we assessed the effects of open label non-mandatory transitioning from originator etanercept (ENB) to biosimilar SB4 on drug survival and effectiveness in rheumatic diseases in daily practice.
Methods: In 2016, 642 ENB treated patients were asked to transition to SB4 by a structured implementation strategy with opt-out option. Consenting patients were eligible for inclusion in our study [BIO-SPAN]. ENB treated patients in 2014 were recruited as historical cohort. The 6-months retention rate in the two cohorts was compared. Multivariable Cox regression analysis was used to calculate HR for discontinuation adjusted for baseline characteristics (age/gender/diagnosis/ENB treatment duration/ENB dose interval/csDMARD/CRP) and with a robust variance estimator to account for repeated subjects. Reasons for discontinuation and change in DAS28-CRP, BASDAI and CRP after 6 months were assessed.
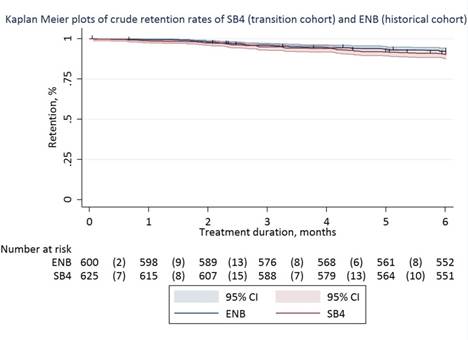
Results: 635 (99%) patients agreed to transition to SB4 of whom 625 patients (433 RA, 128 PsA, 64 AS; 55% women; age 57 (14) years) were included in the transition cohort. 600 patients were included in the historical cohort. Crude 6-months retention rates of SB4 in the transition cohort and ENB in the historical cohort were: 90% (95%CI 88%-93%) vs 92% (95%CI 90%-94%). The transition cohort had a significantly higher relative risk of discontinuation than the historical cohort (adjusted HR 1.57, 95%CI 1.05–2.36). Reasons for discontinuing SB4 (n=60, 10%) and ENB (n=46, 8%) were: lack of effect (43% vs 61%), adverse events (47% vs 28%), malignancy (3% vs 4%), pregnancy (4% vs 4%) and other (3% vs 3%). In the transition cohort, 17 patients restarted ENB, 32 patients switched to another biologic and 11 patients maintained biologic-free. DAS28-CRP, BASDAI and CRP were not statistically different between baseline and month 6 in the transition cohort. Due to a slightly lower baseline CRP (1 [0-5] vs 3 [1-5], p<0.001) and DAS28-CRP (1.9 [1.5-2.6] vs 2.1 [1.6-2.9], p<0.001) changes in CRP (0.5 (12) vs -1.5 (14), p=0.01) and DAS28-CRP (-0.01 (0.93) vs -0.26 (-0.99), p<0.001) at month 6 favored the historical cohort.
Conclusion: Open label non-mandatory transitioning from ENB to SB4 showed a statistically significantly but clinically not relevant lower retention rate compared to a historical cohort, similar to retention seen after mandatory INX transitioning. Non-mandatory transitioning, when executed optimally, might therefore be an attractive alternative to mandatory transitioning.
References: 1Jørgensen KK. Lancet 2017 2Glintborg B. Ann Rheum Dis 2017
To cite this abstract in AMA style:
Tweehuysen L, Huiskes VJB, Van den Bemt (PharmD PhD) BJF, Teerenstra S, van den Hoogen FHJ, van den Ende CHM, Den Broeder AA. Open Label Transitioning from Originator Etanercept to Biosimilar SB4 Compared to Continuing Treatment with Originator Etanercept in a Historical Cohort in Rheumatic Diseases in Daily Practice [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/open-label-transitioning-from-originator-etanercept-to-biosimilar-sb4-compared-to-continuing-treatment-with-originator-etanercept-in-a-historical-cohort-in-rheumatic-diseases-in-daily-practice/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/open-label-transitioning-from-originator-etanercept-to-biosimilar-sb4-compared-to-continuing-treatment-with-originator-etanercept-in-a-historical-cohort-in-rheumatic-diseases-in-daily-practice/