Session Information
Date: Tuesday, November 7, 2017
Title: Pediatric Rheumatology – Clinical and Therapeutic Aspects Poster III: Juvenile Arthritis
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Etanercept (ETN) was the first biologic approved for use in the treatment of patients with polyarticular-course juvenile idiopathic arthritis (JIA),1 and is now indicated in other JIA subcategories.2 Evidence from registry studies and real-world data suggest ETN is favored as a first-line biologic therapy in clinical practice in patients with JIA;3,4 however, the factors associated with long-term retention of ETN in this population have been little explored. Objectives: To evaluate retention rates up to 6 years in ETN-treated pediatric patients in Canada.
Methods: A retrospective cohort study was conducted using longitudinal prescription drug claims data from QuintilesIMS Private Drug Plan database (PDP), Ontario Public Drug Plan database (OPDP), and Quebec Public Drug Plan database. Between 07/2003 and 01/2011, biologic-naïve patients (ie, patients with no biologic treatment in the preceding 12 months) who initiated ETN, were identified and followed for 72 months. Disease indications were inferred through patient drug history. 12-month retention rates were evaluated in 1-year increments for all patients retained on therapy, and compared with the first-year retention rate. Exact 95% confidence intervals (CI) were calculated. McNemar’s test was used to assess the difference between two correlated proportions, where these were based on the same sample of subjects, with reference to year-1 retention. This analysis assessed pediatric patients (ie, age 2-16 years at time of first prescription).
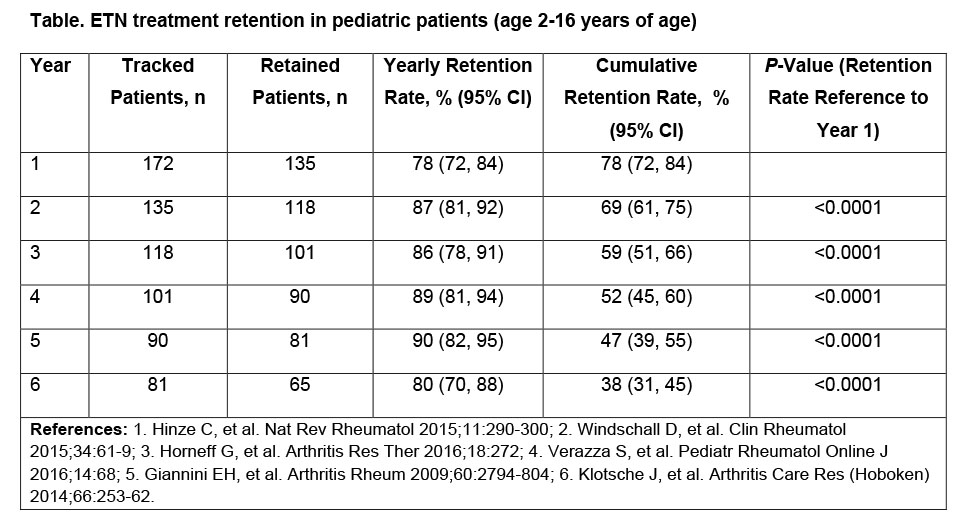
Results: The study identified 172 ETN-treated pediatric patients across Canada, who initiated therapy during the selection period. 67% were female; 94% were diagnosed with JIA. Private claims accounted for 69% of ETN-treated pediatric patients, and 49% of the patient population were from Ontario. Of the 152 patients covered by PDP and OPDP, 45% and 55% were aged 2-11 years and 12-16 years at initiation, respectively. 12-month ETN retention rates increased following their first year on therapy. 78% of patients were retained at year 1; 12-month retention rates through years 2-6 are shown in the Table. Retention rates for the corresponding periods in the adult population (>18 years) were: 66%, 79%, 82%, 84%, 83%, and 79%. 38% of pediatric patients remained on ETN treatment for the entire 72-months’ study. 66 patients switched treatments after discontinuing ETN, with 44% doing so more than once during the 6-year period.
Conclusion: Pediatric patients treated with ETN demonstrated significantly higher retention rates after the first year, with a considerable proportion of patients continuing for 6 years; many who discontinued ETN and subsequently switched treatment did not remain on their first-choice treatment. Further analysis of the reasons for ETN treatment discontinuation may assist in identifying measures to support patients in maintaining treatment to achieve sustained clinical benefit5 and quality of life.6
To cite this abstract in AMA style:
Khraishi MMM, Millson B, Woolcott J, Jones H, Marshall L. Etanercept (Enbrel®) Treatment Retention in the Sub-Population of Pediatric Patients from a Retrospective Cohort Study Using Canadian Claims-Level Data [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/etanercept-enbrel-treatment-retention-in-the-sub-population-of-pediatric-patients-from-a-retrospective-cohort-study-using-canadian-claims-level-data/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/etanercept-enbrel-treatment-retention-in-the-sub-population-of-pediatric-patients-from-a-retrospective-cohort-study-using-canadian-claims-level-data/