Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: In a recent proof-of-concept (PoC) trial it was shown that secukinumab, a fully human IgG1k anti-IL17A monoclonal antibody, significantly improved clinical signs and symptoms of patients with active ankylosing spondylitis (AS). Magnetic resonance images (MRI) of these patients showed reduction of spinal inflammation at week (wk) 6 and wk 28 after initiation of treatment. Here we report on a subgroup of patients (N=13) who entered the open label extension study, received and completed treatment and had MRI assessments at wk 28 and up to 24 months.
Methods: In the 28-wk PoC study, 27/30 patients had sequential MRI studies, 22 had received secukinumab 2×10 mg/kg administered intravenously 3 wks apart, and 5 patients had been randomized to placebo. Of those 27 patients, 20 entered the extension study. 13 of these 20 patients had MRI data at wk 94. Of these 13, ten had been treated with secukinumab and 3 with placebo in the core study. In the extension study, all received secukinumab 14×3 mg/kg administered 4 wks apart. MRIs (T1 and STIR) were rescored for this study by analyzing the images from baseline (BL), wk 28 and wk 94. All MRIs were analyzed together by an independent blinded reader using the ASspiMRI-a scoring system, and also recording the amount of vertebral edges (VEs) which showed inflammation or fatty degeneration at the different time points.
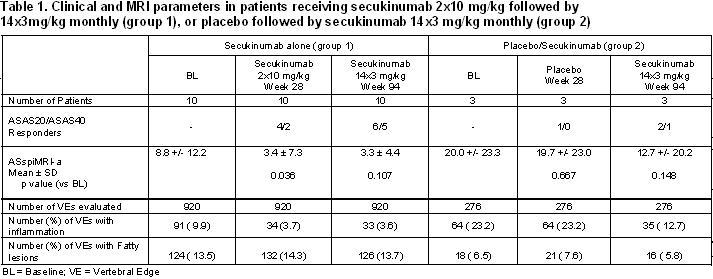
Results: All 13 patients completed the study. For patients receiving 2×10 mg/kg secukinumab followed by 14×3 mg/kg (n=10, group 1), or receiving placebo followed by secukinumab 14×3 mg/kg (n=3, group 2), the ASAS20 and ASAS40 responses are shown in Table 1. In group 1, ASspiMRI-a scores were reduced in comparison to BL at wk 28 – similar to the results of the core study – and these reduced scores were sustained at a similar level at wk 94 (Table 1). Of the 920 VEs evaluated, the proportion of inflammatory lesions was reduced from 9.9% (N=91) at BL to 3.7% (N=34) at wk 28 and 3.6% (N=33) at wk 94. There was no increase in the number of VEs with fatty lesions between BL and wk 28 and 94 (Table 1). In the 3 patients who had initially received placebo and who were then switched to secukinumab at wk 28 (group 2), MRI inflammatory scores at wk 94 also improved (Table 1).
Conclusion: This exploratory MRI analysis shows that the IL-17 inhibitor secukinumab may reduce spinal inflammation and this effect may be sustained for up to 24 months using a lower dose in the maintenance compared to induction phase. Interestingly, there was no change in the amount of fatty lesions in the patients treated with secukinumab – this differs from what was recently reported for the treatment with TNF-blockers. Whether this has an impact on radiographic progression needs to be studied in future trials.
Disclosure:
X. Baraliakos,
None;
J. Braun,
None;
D. D. Laurent,
Novartis Pharma AG,
3;
D. L. Baeten,
Grant / Research support from: Abbott Immunology Pharmaceuticals; Pfizer Inc; Centocor, Inc,
2;
D. van der Heijde,
Abbott, Amgen, AstraZeneca, BMS, Centocor, Chugai, Eli-Lilly, GSK, Merck, Novartis, Otsuka, Pfizer, Roche, Sanofi-Aventis, Schering-Plough, UCB, Wyeth,
2,
Abbott, Amgen, AstraZeneca, BMS, Centocor, Chugai, Eli-Lilly, GSK, Merck, Novartis, Otsuka, Pfizer, Roche, Sanofi-Aventis, Schering-Plough, UCB, Wyeth,
5;
J. Sieper,
None;
P. Emery,
None;
I. B. McInnes,
AstraZeneca, BMS, Centocor, GSK, Merck, Novartis, Novo Nordisk, Pfizer, Roche, UCB,
2,
AstraZeneca, BMS, Centocor, GSK, Merck, Novartis, Novo Nordisk, Pfizer, Roche, UCB,
5;
J. M. van Laar,
None;
R. Landewe,
Abbott, Amgen, AstraZeneca, BMS, Centocor, GSK, Merck, Novartis, Pfizer, Roche, Schering-Plough, UCB, Wyeth,
2,
Abbott, Amgen, AstraZeneca, BMS, Centocor, GSK, Merck, Novartis, Pfizer, Roche, Schering-Plough, UCB, Wyeth,
5;
P. Wordsworth,
Abbott Laboratories; Merck Pharmaceuticals,
8;
J. Wollenhaupt,
None;
H. Kellner,
None;
A. Wright,
Novartis Pharma AG, Basel, Switzerland,
3;
F. Vandenhende,
None;
K. Radford,
Novartis Institutes for BioMedical Research,
3;
B. Borah,
Novartis Institutes for BioMedical Research,
3;
H. Wolfgang,
Novartis Institutes for BioMedical Research,
3.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-inhibition-of-interleukin-il-17a-with-secukinumab-improves-clinical-symptoms-and-reduces-spinal-inflammation-as-assessed-by-magnetic-resonance-imaging-in-patients-with-ankylosing-spondylit/