Session Information
Date: Monday, November 6, 2017
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy II: Trials Therapy
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Fractalkine (CX3CL1/FKN) is a chemokine that regulates chemotaxis and adhesion of CX3C chemokine receptor 1 (CX3CR1)-expressing inflammatory cells. We previously presented the interim results of a 12-week treatment phase during phase 1/2, open-label, multiple ascending dose clinical trial of E6011, a novel humanized anti-FKN monoclonal antibody, in Japanese active rheumatoid arthritis (RA) patients1. This is the first report of the 52-week safety, pharmacokinetics and efficacy of this clinical trial (NCT02196558).
Methods: Japanese patients with active RA who have shown inadequate responses or intolerance to methotrexate (MTX) or TNF inhibitor received E6011 at week 0, 1, 2, and then every 2 weeks for up to week 10 during the Treatment Phase. If no safety concerns were raised, subjects who showed a ≥ 20% improvement in both tender and swollen joint counts were given the option to receive 20 additional biweekly administrations at the same dose in the Extension Phase; 52 weeks in total.
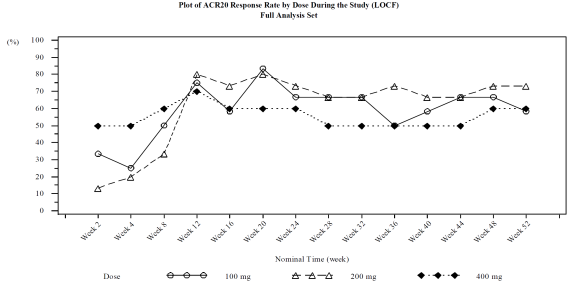
Results: Twelve, 15, and 10 subjects were enrolled in the 100, 200, and 400 mg cohorts, respectively. Of these subjects, a total of 28 subjects were entered in the Extension Phase with 11, 11 and 6 subjects in the 100, 200, and 400 mg cohorts, respectively. As a result, repeated dose of E6011 was found safe and well tolerated during the Extension Phase. The incidence of AE, treatment‑related AE and SAE was 83.8%, 48.6% and 13.5%, respectively. There were no severe AE or deaths, and no significant differences were observed in the incidence or severity of AE across the cohorts. After starting multiple dose administration of E6011, the steady state was achieved at week 2, and was held up to week 52. ACR 20 response rates at Week 12 and 52 (LOCF) were 75.0% and 58.3%, 80.0% and 73.3%, and 70.0% and 60.0% in the 100, 200, and 400 mg cohort respectively.
Conclusion: E6011 was safe and well tolerated, and demonstrated durable efficacy for 52-week in active RA with an inadequate response or intolerance to MTX or TNF inhibitor therapies. These results support the continuation of E6011 clinical study in phase 2 trials during which an optimal clinical dose and further safety and efficacy thresholds should be confirmed in a placebo controlled double-blinded manner.
Reference:
1. 2015 ACR/ARHP Annual Meeting. Abstract Number: 13L
To cite this abstract in AMA style:
Tanaka Y, Takeuchi T, Umehara H, Nanki T, Yasuda N, Tago F, Kawakubo M, Kitahara Y, Hojo S, Kawano T, Imai T. Safety, Pharmacokinetics, and Efficacy of E6011, an Anti-Fractalkine Monoclonal Antibody, in a First-in-Patient Phase 1/2 Study on Rheumatoid Arthritis: 52-Week Results [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/safety-pharmacokinetics-and-efficacy-of-e6011-an-anti-fractalkine-monoclonal-antibody-in-a-first-in-patient-phase-12-study-on-rheumatoid-arthritis-52-week-results/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-pharmacokinetics-and-efficacy-of-e6011-an-anti-fractalkine-monoclonal-antibody-in-a-first-in-patient-phase-12-study-on-rheumatoid-arthritis-52-week-results/