Session Information
Date: Monday, November 6, 2017
Title: Osteoporosis and Metabolic Bone Disease – Clinical Aspects and Pathogenesis
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: In the ACTIVE phase III trial, postmenopausal women with osteoporosis were randomized 1:1:1 to abaloparatide (ABL; n=824), blinded placebo (PBO; n=821), or open-label teriparatide (n=818). During ACTIVE, ABL increased BMD and reduced vertebral, nonvertebral, clinical, and major osteoporotic fractures compared to PBO. Women who completed ABL or PBO treatment in ACTIVE were eligible to enroll in an extension study (ACTIVExtend) to receive up to 24 months of open-label alendronate (ALN). The objectives of ACTIVExtend were to provide additional safety information and to evaluate vertebral and nonvertebral fracture endpoints.
Methods: The ACTIVExtend study enrolled 558 women from the original ABL group and 581 from the PBO group of the ACTIVE study (92% of women who completed ABL or PBO treatment in ACTIVE). Pre-specified endpoints, including vertebral, nonvertebral, clinical, and major osteoporotic fractures, were assessed over the 43-month period from ACTIVE baseline to the end of ACTIVExtend (18 months of ABL or PBO treatment, 1 month for reconsent, and 24 months of ALN treatment). Nonvertebral fracture endpoints were assessed using the Kaplan-Meier (KM) method, proportional hazard model, and logrank test for patients enrolled in ACTIVExtend and for the full ITT population randomized to ABL or PBO treatment in ACTIVE.
Results: During the 43-month period, 5.6% (n=32) of evaluable women sustained a new morphometric vertebral fracture in the PBO followed by ALN (PBO/ALN) group compared to 0.9% (n=5) in the ABL/ALN group, an 84% relative risk reduction (p<0.0001).
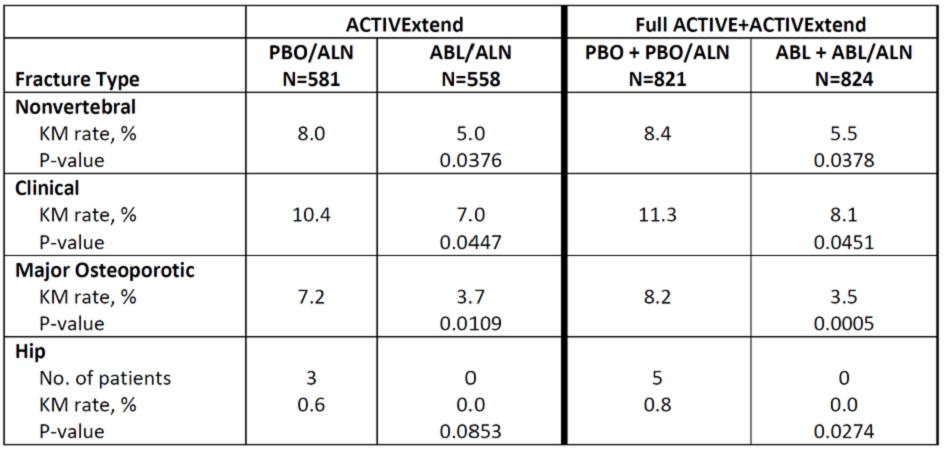
The Table shows the KM rates of nonvertebral fracture endpoints over 43 months from ACTIVE baseline through the end of ACTIVExtend for both the ACTIVExtend cohorts as well as the full ACTIVE ITT population.
The incidence of adverse events (AEs) including severe and serious AEs during ALN treatment period was similar for both study groups. The most common AEs were arthralgia, URI, and back pain. No cases of atypical femoral fracture or osteonecrosis of the jaw were reported.
Conclusion: In the ACTIVExtend study, administration of ABL for 18 months followed by ALN for 24 months resulted in sustained vertebral and nonvertebral fracture reduction compared to PBO followed by ALN. In the full randomized ACTIVE ITT population, over 43 months, in the ABL + ABL/ALN groups, there was a significant reduction in the incidence of vertebral and non-vertebral fractures as well as hip fractures compared to the PBO + PBO/ALN groups.
Table. Kaplan-Meier rates of nonvertebral fracture endpoints during the 43-month analysis period (from ACTIVE baseline through the end of ACTIVExtend).
To cite this abstract in AMA style:
Saag K, Miller PD, Cosman F, Fitzpatrick LA, Hattersley G, Gut R, Mitlak B, Bilezikian JP, Dore RK. Persistent Fracture Reduction with Abaloparatide-SC (TYMLOS™) Followed By 24 Months of Alendronate [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/persistent-fracture-reduction-with-abaloparatide-sc-tymlos-followed-by-24-months-of-alendronate/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/persistent-fracture-reduction-with-abaloparatide-sc-tymlos-followed-by-24-months-of-alendronate/