Session Information
Date: Monday, November 6, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment II
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Psoriatic arthritis (PsA) disease activity score (PASDAS) assessing multiple facets of PsA was demonstrated to distinguish treatment effect, perform better in statistical terms than traditional joint-only indices1 and could be used as a treatment target in clinical trials in PsA. Secukinumab significantly improved the signs and symptoms of PsA over 104 weeks (wks) in the FUTURE 2 study.2 This post-hoc analysis assessed the ability of secukinumab to achieve low disease activity (LDA) or remission (REM) using PASDAS through 104 wks in the FUTURE 2 study.
Methods: FUTURE 2 study design has been previously published.2 PASDAS index is derived from physician’s global VAS, patient’s global VAS, SF-36 PCS, tender and swollen joint counts, Leeds enthesitis count, dactylitis count and CRP level with validated cut-points for high disease activity (HDA ≥5.4), moderate disease activity (3.2< MoDA <5.4), 1.9< LDA ≤3.2 and REM ≤1.9.3 PASDAS was assessed in the overall population and in patients (pts) stratified by prior anti-TNF use (naïve vs inadequate response [IR]) and disease duration (≤2 years vs >2 years since first PsA diagnosis) and reported using mutually exclusive categories at group level and as observed analysis.
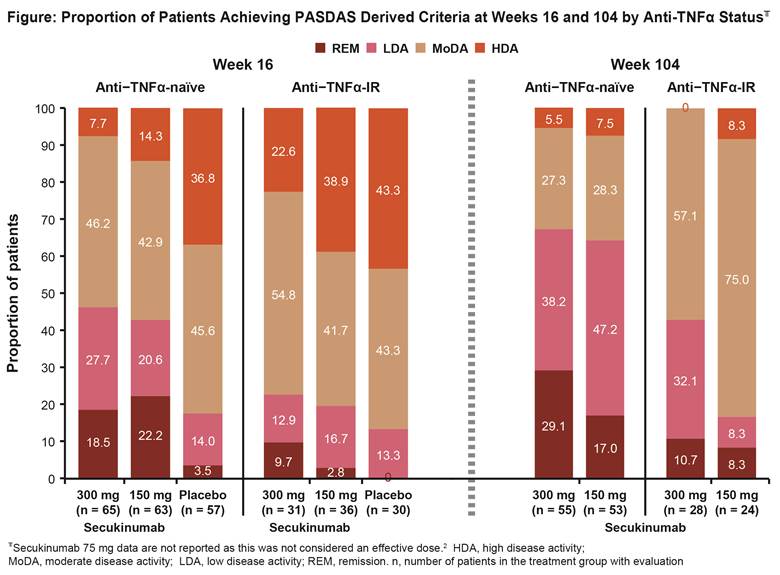
Results: PASDAS score at baseline was similar across the three treatment groups. In the overall population at Wk 16, PASDAS REM, LDA, and MoDA were achieved in: 15.6%, 22.9%, and 49.0% of pts, respectively, treated with secukinumab 300 mg; 15.2%, 19.2%, and 42.4%, respectively, with secukinumab 150 mg group vs. 2.3%, 13.8%, and 44.8%, respectively with placebo (PBO). At Wk 104, REM was achieved in 22.9% and 14.3% pts treated with secukinumab 300 and 150 mg, respectively. The proportion of pts achieving PASDAS derived criteria at Wks 16 and 104 by anti-TNF status is reported in the figure. The proportion of pts achieving PASDAS REM/LDA at Wks 16 and 104 was similar, irrespective of time since first diagnosis, for both secukinumab doses.4 Secukinumab treated pts achieving PASDAS REM had significantly greater improvements in function, physical and mental health quality of life, and fatigue compared to HDA through Wk 104.5
Conclusion: PASDAS REM and LDA were achieved in 38.5% and 34.4% pts treated with secukinumab 300 and 150 mg, respectively, vs 16.1% in placebo group at Wk 16, with approximately 50% pts achieving PASDAS REM and LDA in both secukinumab groups at Wk 104. A higher proportion of anti–TNF-naïve pts treated with secukinumab achieved PASDAS REM or LDA than anti–TNF-IR through Wk 104. Secukinumab treated pts achieving PASDAS REM had significantly greater improvements in function, quality of life, and fatigue.
References: 1. Helliwell PS and Kavanaugh A. Arth Care and Res. 2014;66:749-56; 2.McInnes IB, et al. Arthritis Rheumatol. 2016;68 (suppl 10); 3.Coates LC and Helliwell PS. J Rheumatol. 2016;43:371-5; 4. Coates LC, et al. EULAR 2017 abstract SAT0462 ; 5. Coates LC, et al. EULAR 2017 abstract AB0772.
To cite this abstract in AMA style:
Coates LC, Gladman DD, Nash P, FitzGerald O, Kavanaugh A, Rasouliyan L, Pricop L, Ding K, Gaillez C. Secukinumab Achievement of Psoriatic Arthritis Disease Activity Score (PASDAS) Related Remission: 2-Year Results from a Phase 3 Study [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/secukinumab-achievement-of-psoriatic-arthritis-disease-activity-score-pasdas-related-remission-2-year-results-from-a-phase-3-study/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/secukinumab-achievement-of-psoriatic-arthritis-disease-activity-score-pasdas-related-remission-2-year-results-from-a-phase-3-study/